RSM Process Optimization of Biodiesel Production from Waste Cooking Palm Oil in the Presence of SO3H-PSC Catalysts †
Abstract
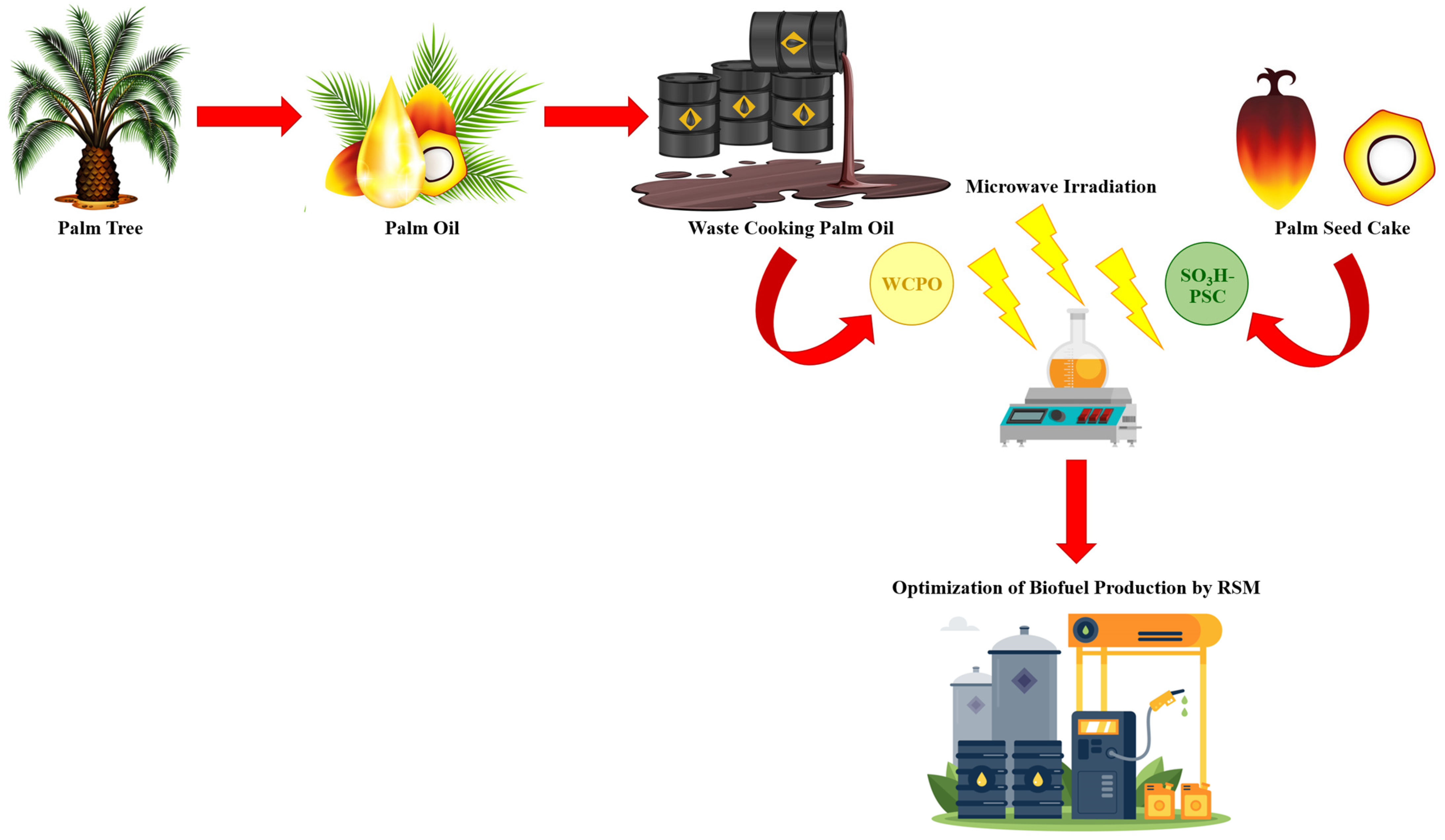
1. Introduction
2. Materials and Methods
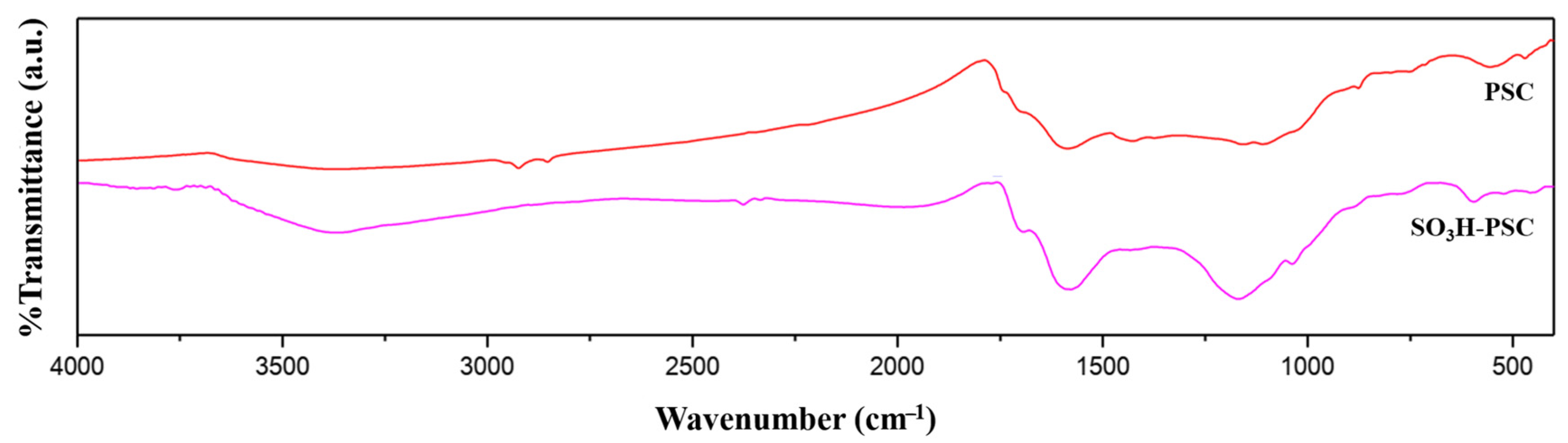
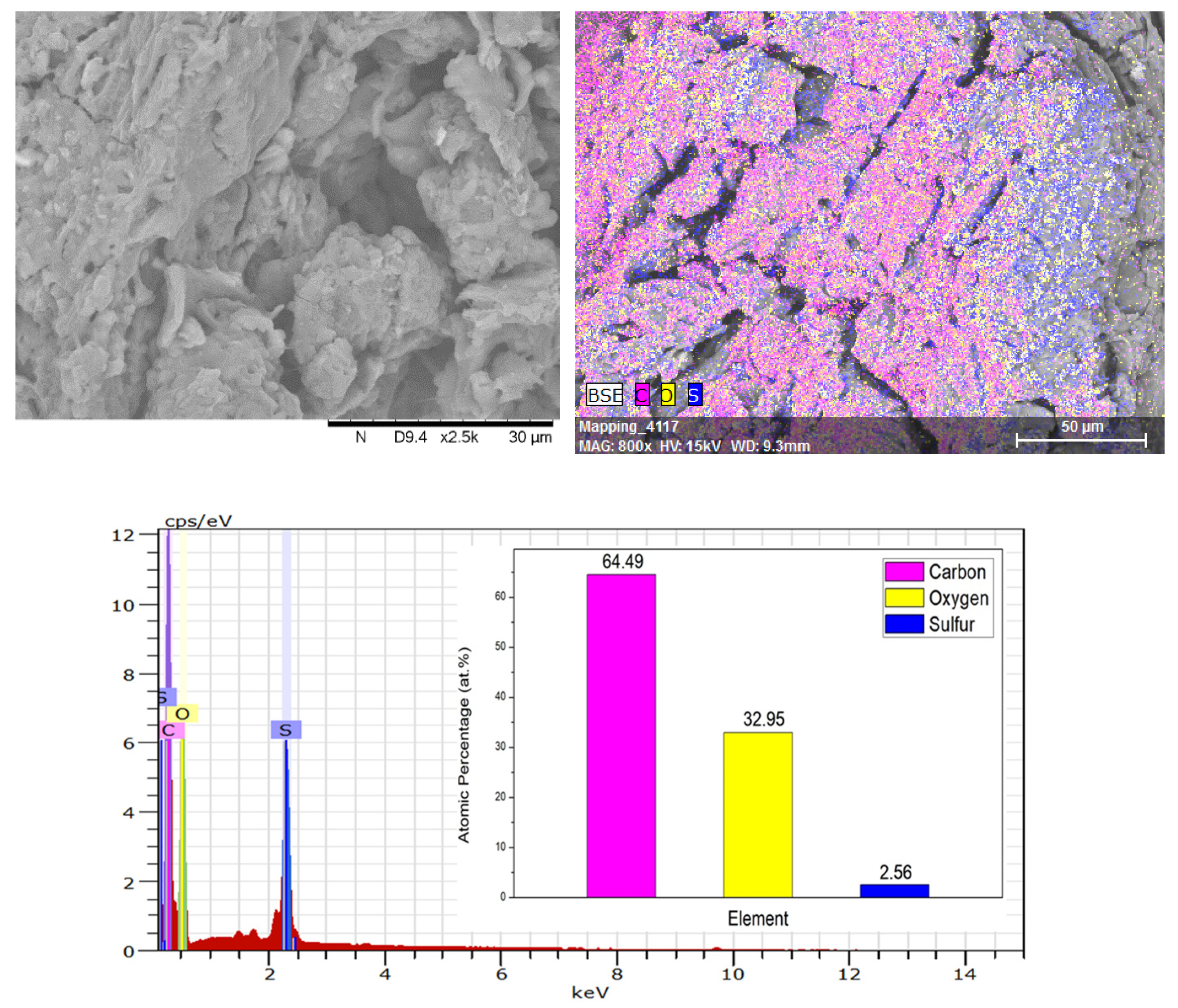
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngomade, S.B.L.; Tchuifon, R.D.T.; Tagne, R.F.T.; Ngueteu, M.L.T.; Patai, H.M.; Nche, G.N.-A.; Anagho, S.G. Optimization by response surface methodology of biodiesel production from Podocarpus falcatus oil as a Cameroonian novel nonedible feedstock. J. Chem. 2022, 2022, 3786602. [Google Scholar] [CrossRef]
- Kolakoti, A.; Jha, P.; Mosa, P.R.; Mahapatro, M.; Kotaru, T.G. Optimization and modelling of mahua oil biodiesel using RSM and genetic algorithm techniques. MME 2020, 6, 134–146. [Google Scholar] [CrossRef]
- Silva, G.F.; Camargo, F.L.; Ferreira, A.L.O. Application of response surface methodology for optimization of biodiesel production by transesterification of soybean oil with ethanol. Fuel Process. Technol. 2011, 92, 407–413. [Google Scholar] [CrossRef]
- Buasri, A.; Loryuenyong, V. The new green catalysts derived from waste razor and surf clam shells for biodiesel production in a continuous reactor. Green Process. Synth. 2015, 4, 389–397. [Google Scholar] [CrossRef]
- Rashid, U.; Ahmad, J.; Ibrahim, M.L.; Nisar, J.; Hanif, M.A.; Shean, T.Y.C. Single-pot synthesis of biodiesel using efficient sulfonated-derived tea waste-heterogeneous catalyst. Materials 2019, 12, 2293. [Google Scholar] [CrossRef] [PubMed]
- Farabi, M.S.A.; Ibrahim, M.L.; Rashid, U.; Taufiq-Yap, Y.H. Esterification of palm fatty acid distillate using sulfonated carbon-based catalyst derived from palm kernel shell and bamboo. Energy Convers. Manag. 2019, 181, 562–570. [Google Scholar] [CrossRef]
- Bazargan, A.; Kostić, M.D.; Stamenković, O.S.; Veljković, V.B.; McKay, G. A calcium oxide-based catalyst derived from palm kernel shell gasification residues for biodiesel production. Fuel 2015, 150, 519–525. [Google Scholar] [CrossRef]
- Akinfalabi, S.-I.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Synthesis of biodiesel from palm fatty acid distillate using sulfonated palm seed cake catalyst. Renew. Energ. 2017, 111, 611–619. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. A novel biobased heterogeneous catalyst derived from Musa acuminata peduncle for biodiesel production—Process optimization using central composite design. Energy Convers. Manag. 2019, 189, 118–131. [Google Scholar] [CrossRef]
- Buasri, A.; Loryuenyong, V. Continuous production of biodiesel from rubber seed oil using a packed bed reactor with BaCl2 impregnated CaO as catalyst. Bull. Chem. React. Eng. Catal. 2018, 13, 320–330. [Google Scholar] [CrossRef]
- Ngige, G.A.; Ovuoraye, P.E.; Igwegbe, C.A.; Fetahi, E.; Okeke, J.A.; Yakubu, A.D.; Onyechi, P.C. RSM optimization and yield prediction for biodiesel produced from alkali-catalytic transesterification of pawpaw seed extract: Thermodynamics, kinetics, and multiple linear regression analysis. Digit. Chem. Eng. 2023, 6, 100066. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, N. Process optimization for production of biodiesel from orange peel oil using response surface methodology. Energ. Source. Part A 2021, 43, 727–737. [Google Scholar] [CrossRef]
- Buasri, A.; Lukkanasiri, M.; Nernrimnong, R.; Tonseeya, S.; Rochanakit, K.; Wongvitvichot, W.; Masa-ard, U.; Loryuenyong, V. Rapid transesterification of Jatropha curcas oil to biodiesel using novel catalyst with a microwave heating system. Korean J. Chem. Eng. 2016, 33, 3388–3400. [Google Scholar] [CrossRef]


| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Catalyst quantity (A), wt.% | 3 | 5 | 7 |
| Methanol/WCPO mole ratio (B), mol/mol | 12 | 15 | 18 |
| Reaction time (C), min | 5 | 7 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buasri, A.; Sirikoom, P.; Pattane, S.; Buachum, O.; Loryuenyong, V. RSM Process Optimization of Biodiesel Production from Waste Cooking Palm Oil in the Presence of SO3H-PSC Catalysts. Eng. Proc. 2023, 37, 73. https://doi.org/10.3390/ECP2023-14666
Buasri A, Sirikoom P, Pattane S, Buachum O, Loryuenyong V. RSM Process Optimization of Biodiesel Production from Waste Cooking Palm Oil in the Presence of SO3H-PSC Catalysts. Engineering Proceedings. 2023; 37(1):73. https://doi.org/10.3390/ECP2023-14666
Chicago/Turabian StyleBuasri, Achanai, Phensuda Sirikoom, Sirinan Pattane, Orapharn Buachum, and Vorrada Loryuenyong. 2023. "RSM Process Optimization of Biodiesel Production from Waste Cooking Palm Oil in the Presence of SO3H-PSC Catalysts" Engineering Proceedings 37, no. 1: 73. https://doi.org/10.3390/ECP2023-14666
APA StyleBuasri, A., Sirikoom, P., Pattane, S., Buachum, O., & Loryuenyong, V. (2023). RSM Process Optimization of Biodiesel Production from Waste Cooking Palm Oil in the Presence of SO3H-PSC Catalysts. Engineering Proceedings, 37(1), 73. https://doi.org/10.3390/ECP2023-14666








