Abstract
The purification of perfluoro(7-methylbicyclo[4.3.0]nonane) industrial fractions with a component content above 0.950 in mass fraction is not effective and requires the use of special separation methods. According to experimental data, the separation factor of the initial mixture during distillation with no additional substances is close to 1. At the same time, the addition of acetone (Ac) makes it possible to significantly intensify the process. Ac allowed for obtaining MBCN with a purity higher than 0.998 in mass fraction in one separation cycle, and the degree of recovery was more than 0.85 by weight. The paper presents data on the distribution of components (target product, identified and unidentified impurities of electrochemical fluorination of decalin) between the distillate and bottom product fractions, separation factor, liquid–liquid phase equilibrium in the MBCN-Ac system, and characteristics of the MBCN-Ac heteroazeotrope.
1. Introduction
The manufacture of perfluorodecalin (PFD) in industry occurs in two stages [1]. The first stage is “mild” fluorination, which allows for protecting the carbon chain. The second stage is where the mixture is treated by molecular fluorine to remove the residual partially fluorinated molecules, the content of which is strictly regulated. At the same time, there is a partial destruction of the carbon chain in the molecules of the target component. As part of this process, a number of studies have been published on the structure and nature of impurity components [2,3,4] and the properties of reaction mixture constituents [5,6,7,8]. However, the question of the mechanism of the side reactions and the structure of their products is still open, since the identification of the components required spectral analysis of the samples, which has not yet been obtained in pure form. That is, the reference samples are not available in practice, and data on the spectral and physicochemical properties are not available in the literature, which makes the direct physical and chemical search for intensification methods of the production and purification of these compounds deeply problematic.
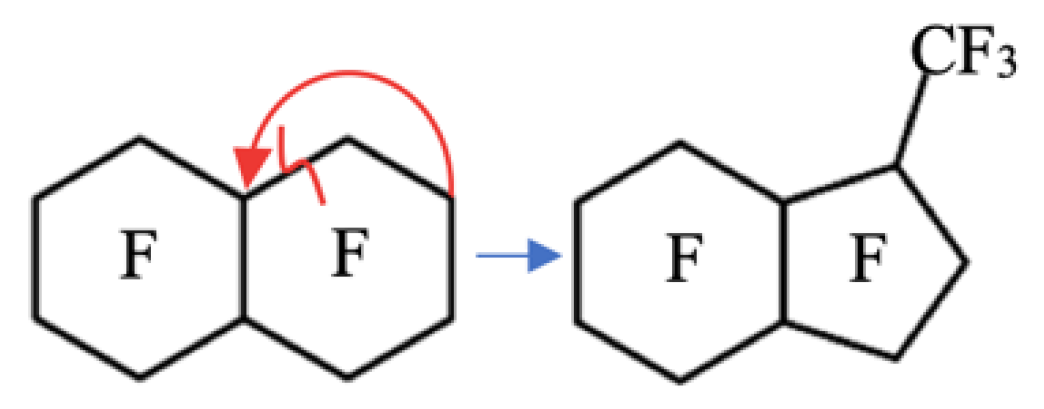
Perfluoro(7-methylbicyclo[4.3.0]nonane) (MBCN) is one of the identified impurities of a closely boiling industrial mixture of products of the configurational and structural isomeric reaction of the electrochemical fluorination of decalin and naphthalene [9]. During the target component production, PFD, there is a partial destruction of the carbon chain followed by a partial radical cycle-closing reaction to form MBCN (Figure 1).
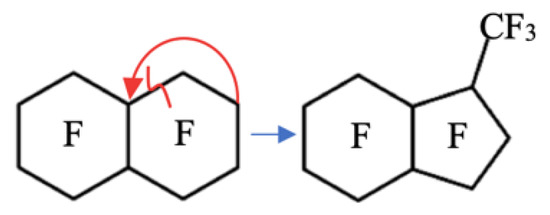
Figure 1.
Mechanism of the side reaction of PFD carbon chain destruction and MBCN formation.
It should be noted that the side perfluorinated alicyclic impurities have their own commercial value. They are used as monomers in the production of lyophobic polymer films, tracers to assess leakage from the sequestration of CO2 in a depleted oil reservoir, and optical elements including lenses where the focal length can be controlled using an electric field [10,11,12,13].
The purpose of this work is the process of distillation purification of the constituent of an industrial mixture of products of electrochemical fluorination of decalin and naphthalene—perfluoro(7-methylbicyclo[4.3.0]nonane).
2. Materials and Methods
As an investigation object, in the present work, the fraction of MBCN with a main component content ranging from 0.950 to 0.975 in mass fraction was considered. The mixture itself with an initial content of the target product of about 0.80 in mass fraction was taken directly from industry. Further, the mixture can be purified by distillation to 0.950 ÷ 0.975 in mass fraction of MBCN. Subsequent separation of the resulting mixture by distillation does not lead to a change in the fractional composition. The impurity content can be divided into the following subsections: identified—PFD (≈0.004 ÷ 0.025 in mass fraction); not identified—main impurity (main imp. ≈ 0.012 ÷ 0.028 in mass fraction); and sum of other impurities (∑ other imp. ≈ 0.0001 ÷ 0.008 in mass fraction; up to 10 compounds).
The heteroazeotropic distillation was used to intensify the separation process. Acetone (Ac) (CAS No 67-64-1, ≥0.995 in mass fraction) and dimethylformamide (DMFA) (CAS No 68-12-2, ≥0.990 in mass fraction) were considered as heteroazeotrope-forming agents (SA). All distillation experiments were performed at atmospheric pressure (P) on a semicommercial batch distillation column with an efficiency of 85 theoretical separation stages. The flow diagram of the unit and its structure are fully described in [14]. During the experiment, distillate was sampled every two hours, with every 8 samples being a fraction of the distillate (Fr). Gas chromatography (GC) and nuclear magnetic resonance (NMR) methods were used for analytical determination of the phase compositions. The initial mixture (F), the distillate fractions (Fr), the total distillate (∑D), and the bottom (W) were analyzed. In the case of heteroazeotropic distillation, the dissolved separating agent was removed from the samples by extraction with water.
Liquid–liquid equilibrium data were obtained by the cloud point method. The composition of each cloud point was determined gravimetrically. All quantities were weighted in an analytical balance (±5 × 10−4 g). The heteroazeotrope characteristic was determined by continuous distillate flow sampling at a 0 reflux ratio value. The obtained fraction was thermostated in a separating flask, and after that, the phases were separated and weighed. The composition of the phases was determined from the data on the liquid–liquid phase equilibrium. For the experiments, a fraction with a content of MBCN ≥0.998 in mass fraction was used.
3. Experimental Results
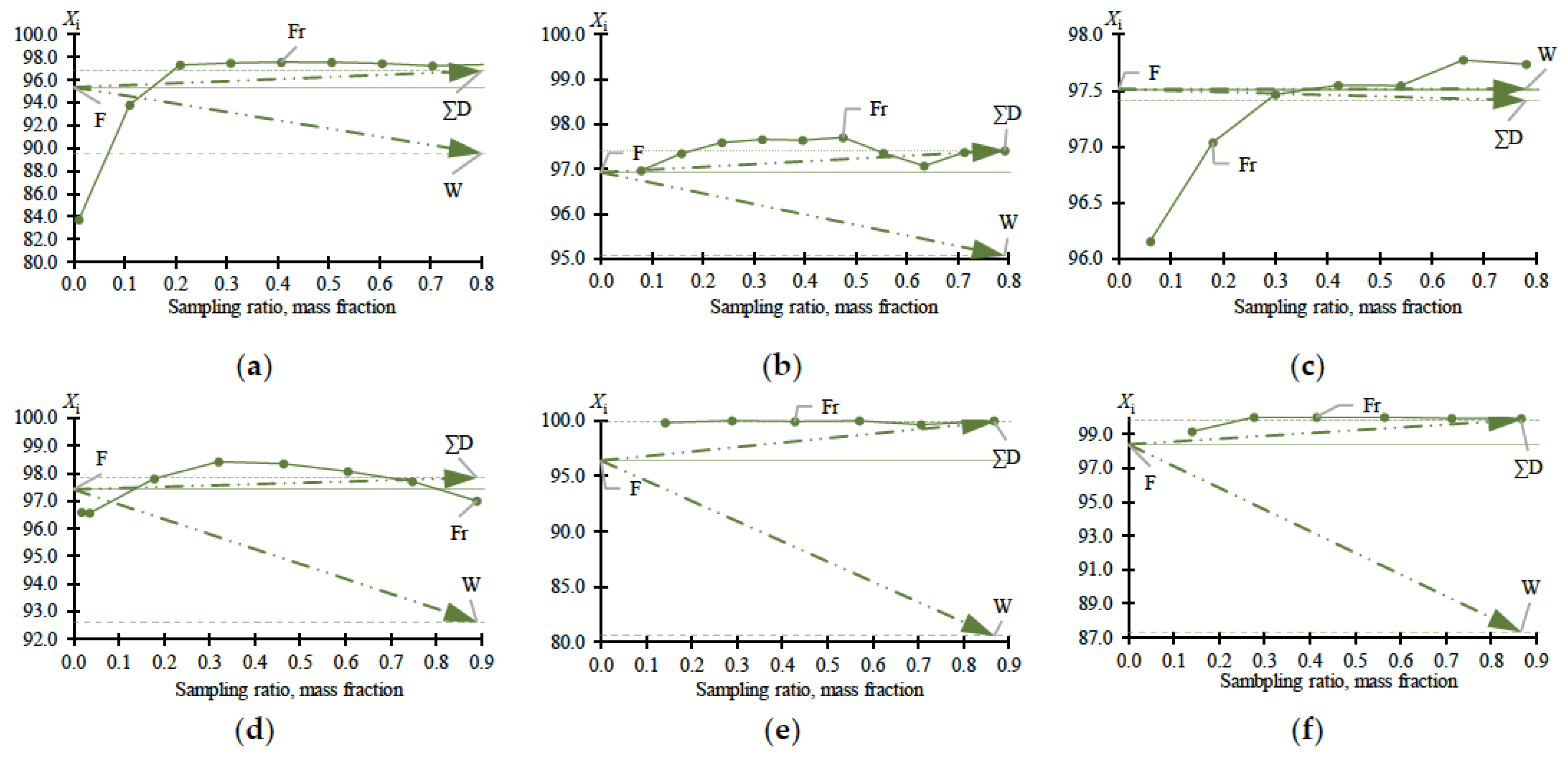
Experimental data on MBCN distillation, including in the presence of heteroazeotrope-forming agents, are shown in Table 1. The data in Table 1 plot the dependences (Figure 2) of distillate fractions (Fr), total distillate (∑D), and bottom (W) compositions versus sampling ratio (Equation (1)):
where —feed weight, g; —the total amount of sampled distillate, g.

Table 1.
Experimental data on MBCN purification by distillation at atmospheric pressure.
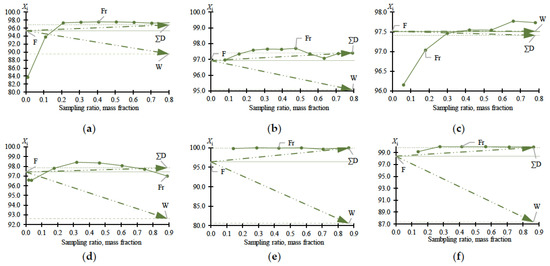
Figure 2.
MBCN content (X, mass %) in samples versus sampling ratio according to Table 1: (a) experiment no. 1; (b) experiment no. 2; (c) experiment no. 3; (d) experiment no. 4; (e) experiment no. 5; (f) experiment no. 6. F—feed; W—bottom; ∑D—total distillate; Fr—fraction composition.
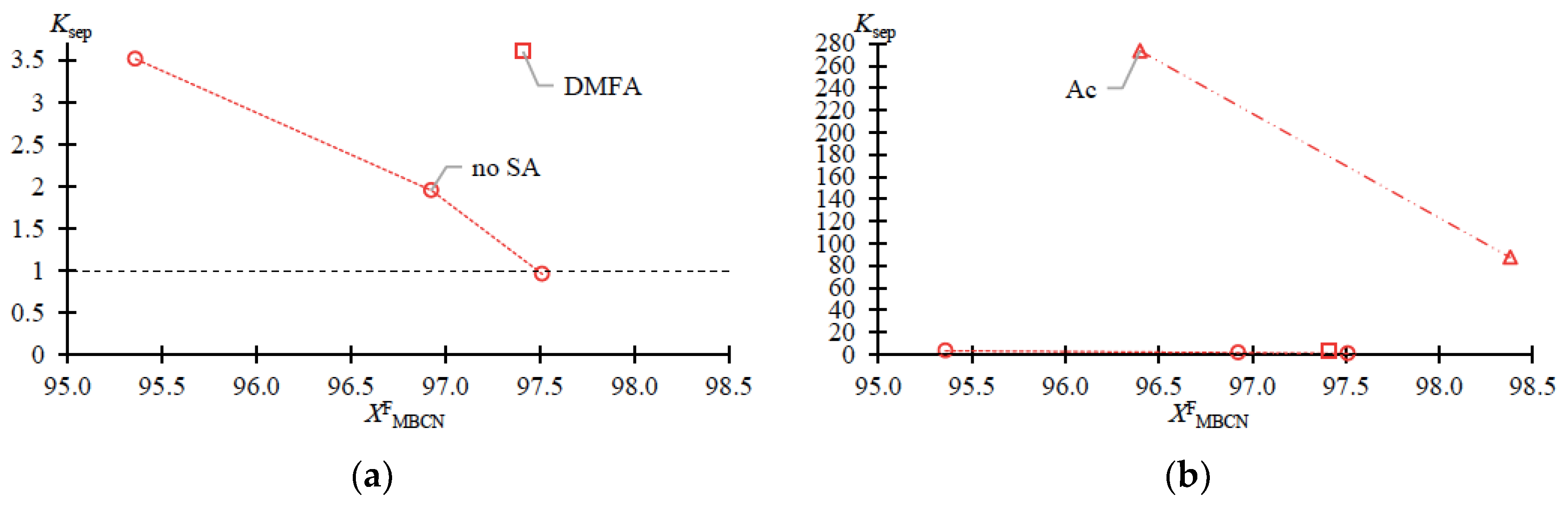
The dependences of the separation ratio () and ratio of enrichment () of MBCN versus the target component content in the feed fraction are shown in Figure 3 and Figure 4, accordingly. (Equation (2)) and (Equation (3)) are calculated according to Table 1:
where —MBCN content, mass %; F—feed; W—bottom; ∑D—total distillate.
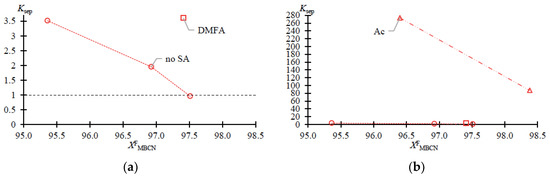
Figure 3.
Separation ratio (Ksep) of MBCN versus target component content in feed fraction (Table 1 data). (a) zoomed in; (b) normal view.
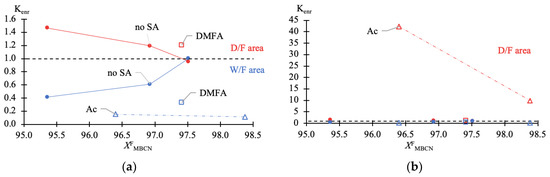
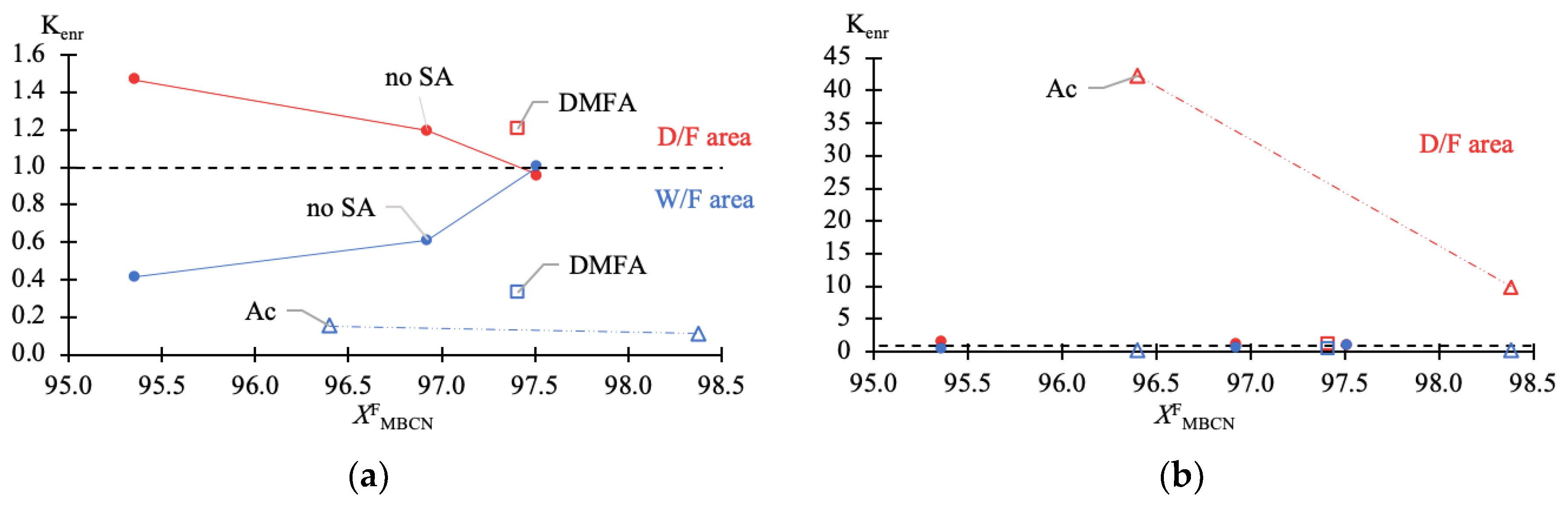
Figure 4.
Ratio of enrichment (Kenr) of MBCN versus target component content in feed fraction (Table 1 data). (a) zoomed-in W/F area (—blue); (b) diminishable D/F area (—red).
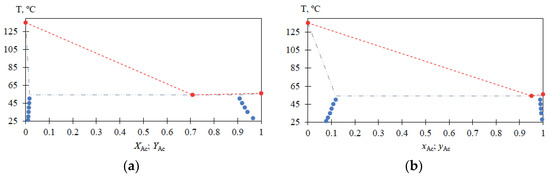
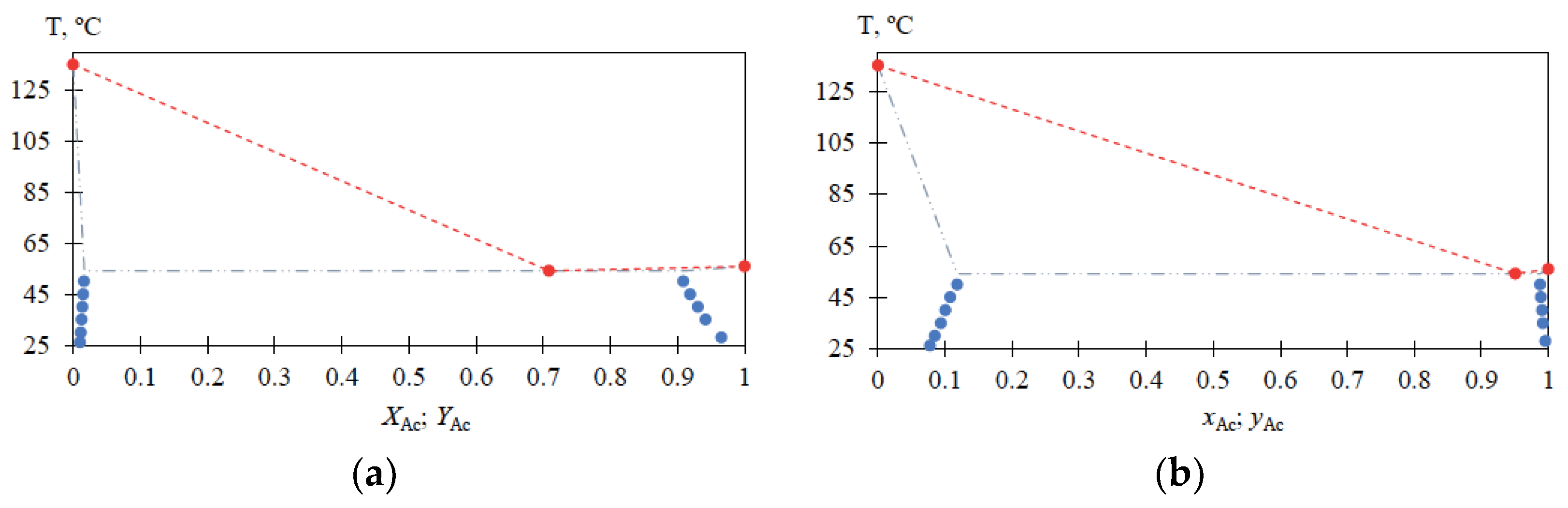
Experimental data on liquid–liquid equilibrium in the MBCN-Ac system for the temperature range (T) from 25 to 50 °C are given in Table 2; the characteristics of the MBCN-Ac heteroazeotrope at atmospheric pressure are given in Table 3; a view of the MBCN-Ac mixture phase diagram according to Table 2 and Table 3 is shown in Figure 5.

Table 2.
Liquid–liquid equilibrium data in MBCN-Ac system at atmospheric pressure, 100.0 kPa.

Table 3.
MBCN-Ac heteroazeotrope composition.
4. Discussion
The data given in Table 1 fully reflect the complex of thermodynamic constraints on the distillation process. The main problem is the withdrawal of PFD and the so-called “main impurity” (still not identified, due to the difficulty of its concentration and purification). From the data in Table 1 and Figure 2a–c, it follows that at relatively low MBCN concentrations in the feed solution, the distillation method allows the fractionation of the mixture and concentration of the target component in distillate. However, when approaching 0.975 in mass fraction of MBCN in the feed solution, this method becomes useless (Ksep → 1; Kenr → 1). The addition of DMFA allows a minor but intensified separation process (Figure 2d): Ksep ≈ 1 → 3.6 (Figure 3); ≈ 1 → 0.33; and ≈ 1 → 1.2 (Figure 4).
Ac is more efficient not only when compared to DMFA (Figure 2f) but also when separating a mixture with a lower (0.964 in mass fraction) concentration of the target component in the feed solution (Figure 2e). Thus, adding Ac makes it possible to obtain an MBCN fraction with a purity higher than 0.998 in mass fraction, and the degree of recovery is quite high, ≥0.85 in mass fraction, in just one process cycle. At = 0.964 ÷ 0.984 in mass fraction, the separation process intensifies by an order of magnitude: Ksep ≈ 1 → 273.46 ÷ 87.55 (Figure 3); ≈ 1 → 0.15 ÷ 0.11; and ≈ 1 → 42.26 ÷ 9.96 (Figure 4).
For the MBCN-Ac system, the liquid–liquid phase equilibrium data are also obtained in the temperature range from 25 to 50 °C (Table 2) and heteroazeotrope characteristics are determined (Table 3). The solubilities of Ac in MBCN (xAc = 0.0778 → 0.1183 mole fraction) and MBCN in Ac (xAc = 0.9956 → 0.9876 mole fraction) increase with increasing temperature from 25 to 50 °C; the heteroazeotrope composition at atmospheric pressure is greatly shifted toward Ac. The presented data (Figure 5) allow for estimating the loss of Ac in the distillate flow and calculating the amount of heteroazeotrope-forming agent required for the process, as well as being essential for the process flowsheet design.
5. Conclusions
This work presents only a part of a large data set on the purification of MBCN from close-boiling impurities and on the separation of the mixture of configurational and structural isomeric reaction products of the electrochemical fluorination of decalin and naphthalene. The major difficulties faced in intensifying the process are the insufficient reference data on the composition of the mixture, the properties of the components present in it, and the topology of the phase diagram of the reaction system. Nevertheless, despite the absence of impurity nomenclature in the present work, it was possible to significantly (by an order of magnitude) increase the efficiency of the MBCN purification process. The effect was achieved by adding a heteroazeotrope-forming agent, Ac. Experimental data on liquid–liquid phase equilibrium were also obtained for the MBCN-Ac binary mixture and the characteristics of the heteroazeotrope at atmospheric pressure were determined.
First, the new experimental data reported in the present work are reference data, the technological solution, and the entry point for further research in terms of the intensification of the process of production and the purification of perfluorinated cycloalkanes.
Author Contributions
Conceptualization, A.V.P., E.V.L. and S.Y.K.; validation, A.V.P., E.V.L. and A.V.K.; formal analysis, A.V.P. and E.V.L.; investigation, A.V.P., E.V.L. and A.V.K.; resources, S.Y.K.; writing—original draft preparation, A.V.P.; writing—review and editing, A.V.P.; visualization, A.V.P.; supervision, A.V.P. and E.V.L.; project administration, A.V.P., E.V.L. and N.N.K.; funding acquisition, A.V.P., E.V.L., S.Y.K. and N.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Russian Science Foundation, project no. 22-29-00791, https://rscf.ru/en/project/22-29-00791/ (accessed on 11 July 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aleshinskii, V.V.; Novikova, M.D.; Shabalin, D.A. Method of Producing Perfluorocycloalkanes. Patent No. RU 2451006 C1, 20 May 2012. [Google Scholar]
- Gervits, L.L.; Snegirov, V.F.; Makarov, K.N.; Galakhov, M.V.; Mukhin, V.Y. Non-Chair Conformation of Cis Isomers of 1,4-Disubstituted Perfluorocyclohexanes. Bull. Acad. Sci. USSR Div. Chem. Sci. 1987, 36, 2664–2665. [Google Scholar] [CrossRef]
- Gervits, L.L. Perfluorocarbon-Based Blood Substitutes Russian Experience. Fluor. Med. 21st Century 1994, 22, 18–21. [Google Scholar]
- Kambur, P.S.; Pashkevich, D.S.; Alekseev, Y.I.; Yampolskii, Y.P.; Alentev, A.Y. Interaction of Perfluorinated Fluids with Fluorine in Gas-Liquid Reactor. Russ. J. Appl. Chem. 2019, 92, 661–666. [Google Scholar] [CrossRef]
- Moshnyaga, A.V.; Khoroshilov, A.V.; Selivanova, D.I.; Aksenova, D.M. Thermodynamics of Dissolved Nitrogen, Nitrous Oxide, and Ammonia in Perfluorodecalin. Russ. J. Phys. Chem. A 2017, 91, 2117–2120. [Google Scholar] [CrossRef]
- Moshnyaga, A.V.; Khoroshilov, A.V.; Semyashkin, M.P.; Mel’nikov, V.V. Density of N2O Solutions in Perfluorodecalin As a Function of Concentration. Russ. J. Phys. Chem. A 2018, 92, 719–723. [Google Scholar] [CrossRef]
- Hassanalizadeh, R.; Nelson, W.M.; Naidoo, P.; Ramjugernath, D. Measurement and Modeling of the Solubility of Tetrafluoromethane in Either Perfluoroheptane or Perfluorodecalin. J. Chem. Eng. Data 2020, 65, 4862–4868. [Google Scholar] [CrossRef]
- Deepika, D.; Pandey, S. Density and Dynamic Viscosity of Perfluorodecalin-Added n-Hexane Mixtures: Deciphering the Role of Fluorous Liquids. Liquids 2023, 3, 48–56. [Google Scholar] [CrossRef]
- Polkovnichenko, A.V.; Lupachev, E.V.; Kisel’, A.V.; Kvashnin, S.Ya.; Kulov, N.N. Perfluoro(7-Methylbicyclo[4.3.0]Nonane) and Perfluoro(Butylcyclohexane): Physicochemical, Thermophysical, and Spectral Data. J. Chem. Eng. Data 2023, 68, 499–517. [Google Scholar] [CrossRef]
- Hynes, A.M.; Shenton, M.J.; Badyal, J.P.S. Plasma Polymerization of Trifluoromethyl-Substituted Perfluorocyclohexane Monomers. Macromolecules 1996, 29, 18–21. [Google Scholar] [CrossRef]
- Wells, A.W.; Diehl, J.R.; Bromhal, G.; Strazisar, B.R.; Wilson, T.H.; White, C.M. The Use of Tracers to Assess Leakage from the Sequestration of CO2 in a Depleted Oil Reservoir, New Mexico, USA. Appl. Geochem. 2007, 22, 996–1016. [Google Scholar] [CrossRef]
- Tuffin, R.; Paari, O.L.; Baker, P.; Brown, C.; Sage, I.C. Material Combination. Patent No. EP3334801B1, 14 July 2016. [Google Scholar]
- Dionisio, K.L.; Phillips, K.; Price, P.S.; Grulke, C.M.; Williams, A.; Biryol, D.; Hong, T.; Isaacs, K.K. The Chemical and Products Database, a Resource for Exposure-Relevant Data on Chemicals in Consumer Products. Sci. Data 2018, 5, 180125. [Google Scholar] [CrossRef] [PubMed]
- Kulov, N.N.; Polkovnichenko, A.V.; Lupachev, E.V.; Rastunova, I.L.; Magomedbekov, E.P. Fractionation of D/H and 18O/16O Water Isotopes in a Packed Distillation Column. Theor. Found. Chem. Eng. 2020, 54, 389–396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).