Bioinformatics Approaches for Structural and Functional Annotation of an Uncharacterized Protein of Helicobacter pylori †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Sequence Retrieval
2.2. Protein Characterization
2.3. Functional Annotation Anticipation
2.4. Prediction of Secondary Structure
2.5. Three-Dimensional Structure Prediction and Validation
3. Results and Discussion
3.1. Protein Sequence Retrieval
3.2. Protein Characterization
3.3. Functional Annotation Prediction
3.4. Secondary Structure Prediction
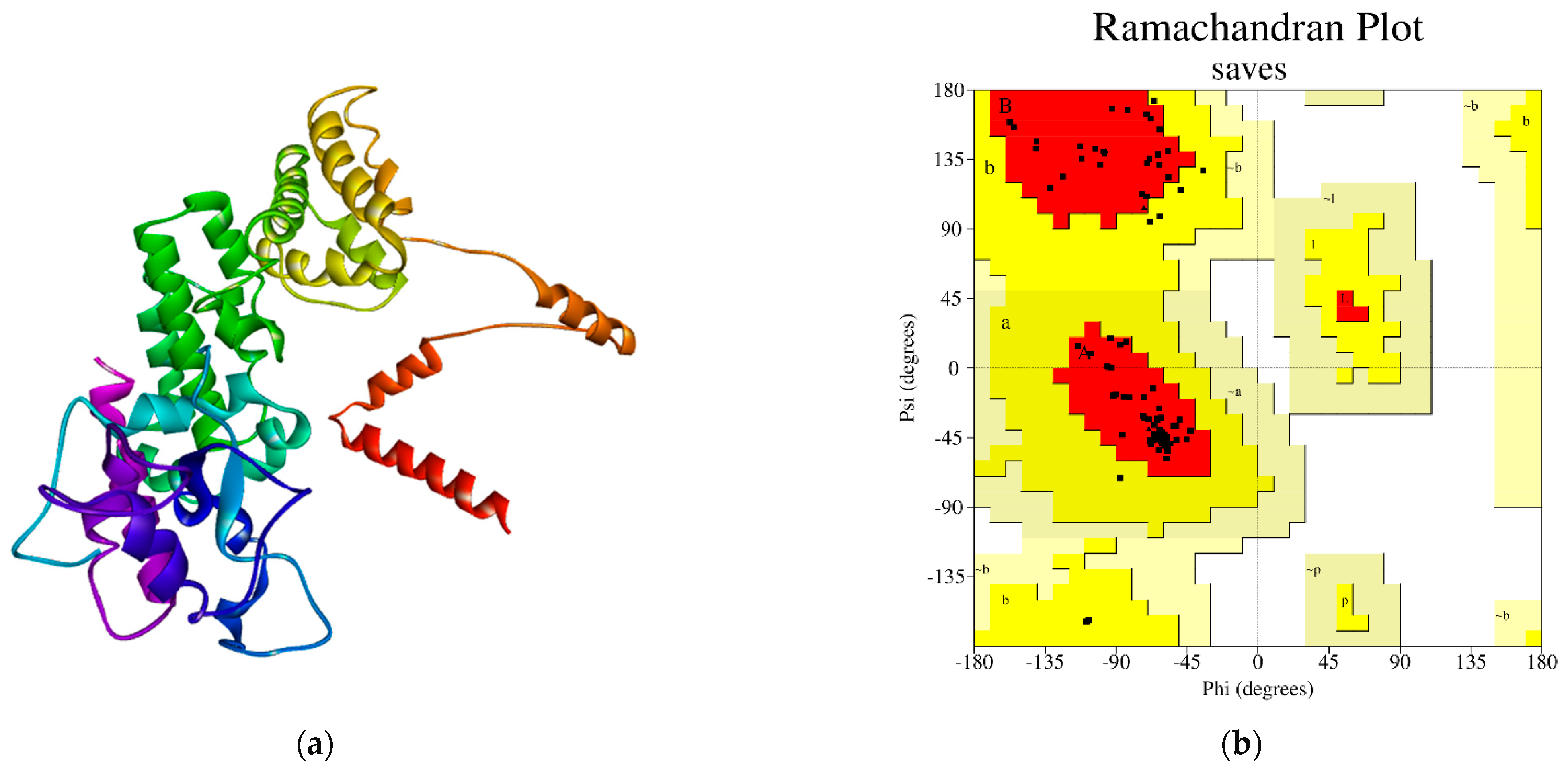
3.5. Three-Dimentional Structure Prediction and Validation of the Selected Protein
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Go, M.F. Review article: Natural history and epidemiology of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2002, 16 (Suppl. S1), 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yamada, N.; Wu, Y.L.; Wen, M.; Matsuhisa, T.; Matsukura, N. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J. Gastroenterol. 2005, 11, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Bazzoli, F.; El-Omar, E.; Graham, D.; Hunt, R.; Rokkas, T.; Vakil, N.; Kuipers, E.J. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut 2007, 56, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Gasbarrini, A.; Polyzos, S.A.; Kountouras, J. Extragastric Diseases and Helicobacter pylori. Helicobacter 2015, 20 (Suppl. S1), 40–46. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M., Jr. Helicobacter pylori: Gastric cancer and beyond. Nature reviews. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Feldman, R.A. Epidemiologic observations and open questions about disease and infection caused by H. pylori. In H. pylori Molecular and Cellular Biology; Horizon Scientific: Wymondham, UK, 2007; pp. 29–51. [Google Scholar]
- Rowland, M.; Kumar, D.; Daly, L.; O’connor, P.; Vaughan, D.; Drumm, B. Low rates of Helicobacter pylori reinfection in children. Gastroenterology 1999, 117, 336–341. [Google Scholar] [CrossRef]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Saikat, A.S.M.; Paul, A.K.; Dey, D.; Das, R.C.; Das, M.C. In-Silico Approaches for Molecular Characterization and Structure-Based Functional Annotation of the Matrix Protein from Nipah henipavirus. Chem. Proc. 2022, 12, 21. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef]
- Saikat, A.S.M.; Islam, R.; Mahmud, S.; Imran, M.A.S.; Alam, M.S.; Masud, M.H.; Uddin, M.E. Structural and Functional Annotation of Uncharacterized Protein NCGM946K2_146 of Mycobacterium Tuberculosis: An In-Silico Approach. Proceedings 2020, 66, 13. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- de Castro, E.; Sigrist, C.J.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef]
- Saikat, A.S.M.; Uddin, M.E.; Ahmad, T.; Mahmud, S.; Imran, M.A.S.; Ahmed, S.; Alyami, S.A.; Moni, M.A. Structural and Functional Elucidation of IF-3 Protein of Chloroflexus aurantiacus Involved in Protein Biosynthesis: An In Silico Approach. BioMed Res. Int. 2021, 2021, 9050026. [Google Scholar] [CrossRef]
- Combet, C.; Blanchet, C.; Geourjon, C.; Deléage, G. NPS@: Network protein sequence analysis. Trends Biochem. Sci. 2000, 25, 147–150. [Google Scholar] [CrossRef]
- Saikat, A.S.M. Computational approaches for molecular characterization and structure-based functional elucidation of a hypothetical protein from Mycobacterium tuberculosis. Genomics Inform. 2023, 21, e25. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Saikat, A.S.M.; Ripon, A. Structure Prediction, Characterization, and Functional Annotation of Uncharacterized Protein BCRIVMBC126_02492 of Bacillus cereus: An In Silico Approach. Am. J. Pure Appl. Biosci. 2020, 2, 104–111. [Google Scholar] [CrossRef]
- Nichols, N.M. Endonucleases. In Current Protocols in Molecular Biology; John Wiley & Sons: Hoboken, NJ, USA, 2011; Chapter 3, Unit 3.12. [Google Scholar] [CrossRef]
- Saikat, A.S.M.; Das, R.C.; Das, M.C. Computational Approaches for Structure-Based Molecular Characterization and Functional Annotation of the Fusion Protein of Nipah henipavirus. Chem. Proc. 2022, 12, 32. [Google Scholar]
- Saikat, A.S.M. An In Silico Approach for Potential Natural Compounds as Inhibitors of Protein CDK1/Cks2. Chem. Proc. 2022, 8, 5. [Google Scholar] [CrossRef]
- Tuncbag, N.; Gursoy, A.; Keskin, O. Prediction of protein-protein interactions: Unifying evolution and structure at protein interfaces. Phys. Biol. 2011, 8, 035006. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | Quantity | Percentage |
|---|---|---|
| Ala (A) | 20 | 4.4% |
| Arg (R) | 15 | 3.3% |
| Asn (N) | 23 | 5.1% |
| Asp (D) | 34 | 7.5% |
| Cys (C) | 7 | 1.6% |
| Gln (Q) | 12 | 2.7% |
| Glu (E) | 41 | 9.1% |
| Gly (G) | 11 | 2.4% |
| His (H) | 11 | 2.4% |
| Ile (I) | 29 | 6.4% |
| Leu (L) | 56 | 12.4% |
| Lys (K) | 54 | 12.0% |
| Met (M) | 10 | 2.2% |
| Phe (F) | 18 | 4.0% |
| Pro (P) | 13 | 2.9% |
| Ser (S) | 29 | 6.4% |
| Thr (T) | 23 | 5.1% |
| Trp (W) | 8 | 1.8% |
| Tyr (Y) | 21 | 4.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.M.; Saikat, A.S.M.; Uddin, M.E. Bioinformatics Approaches for Structural and Functional Annotation of an Uncharacterized Protein of Helicobacter pylori . Eng. Proc. 2023, 37, 61. https://doi.org/10.3390/ECP2023-14671
Alam MM, Saikat ASM, Uddin ME. Bioinformatics Approaches for Structural and Functional Annotation of an Uncharacterized Protein of Helicobacter pylori . Engineering Proceedings. 2023; 37(1):61. https://doi.org/10.3390/ECP2023-14671
Chicago/Turabian StyleAlam, Md. Morshed, Abu Saim Mohammad Saikat, and Md. Ekhlas Uddin. 2023. "Bioinformatics Approaches for Structural and Functional Annotation of an Uncharacterized Protein of Helicobacter pylori " Engineering Proceedings 37, no. 1: 61. https://doi.org/10.3390/ECP2023-14671
APA StyleAlam, M. M., Saikat, A. S. M., & Uddin, M. E. (2023). Bioinformatics Approaches for Structural and Functional Annotation of an Uncharacterized Protein of Helicobacter pylori . Engineering Proceedings, 37(1), 61. https://doi.org/10.3390/ECP2023-14671








