Abstract
This work uses three-dimensional green and biodegradable adsorbent from cellulose nanocrystals and a machine learning technique to simulate and optimise the removal of zinc (II) from synthetic acid mine drainage. The adsorption process was modelled and optimised using three machine learning algorithms: response surface methodology (RSM), adaptive neuro-fuzzy inference system (ANFIS), and artificial neural network (ANN). According to the findings, the created models successfully predicted the adsorption behaviour, with the ANN model performing best with the lowest error rate. The study also looked at the impact of other factors on the adsorption process, such as pH, adsorbent dosage, temperature, and starting concentration. The RSM was used to optimise the process, and the ideal conditions for the maximal zinc (II) removal efficiency were established. The best conditions were established to be an initial pH of 6, an initial concentration of 175 mg/L, a contact period of 100 min, and a sorbent dosage of 6 mg/L. The results show that the created three-dimensional adsorbent and machine learning approach, namely, the ANFIS model, are promising strategies for removing zinc (II) from acid drainage. The study’s findings might help develop cost-effective and efficient systems for treating polluted water supplies.
1. Introduction
Environmental pollution results from the global growth of companies and manufacturing plants. One of the primary sources of pollution is the excessive release of heavy metals from factories that produce paint, plastics, textiles, batteries, and electroplating. The wastewater from these industries contains heavy metals, such as chromium, lead, mercury, cadmium, zinc, nickel, and copper [1]. These heavy metals have high toxicity and are not biodegradable. Industries frequently release their effluents into the environment without pretreatment, creating a significant risk to human life and the ecosystem [2]. Heavy metals must be removed from industrial effluents because they are hazardous. Industries employ various methods to remove and recover heavy metals from discharges, including filtration, membrane separation, chemical precipitation, ion exchange, coagulation, reverse osmosis, and adsorption [3].
The need for nanoparticles has grown as they have become more efficient and innovative in adsorbents. According to one description, superabsorbent hydrogels are three-dimensional cross-linked polymers that can absorb and retain large quantities of aqueous materials [4]. In recent years, polymeric adsorbents have been widely used to remove dyes. The most prevalent processes for removing heavy metal ions from water include hydrogen bonding, electrostatic attraction, and ion exchange between heavy metal ions [5]. As cellulose nanocrystals are one of the most easily obtained and economical polysaccharides with many hydroxyl groups, they have been widely used in recent years to produce new hydrogels for specific environmental applications [6].
The response surface methodology (RSM) is a statistical technique for process optimisation that differs from one component at a time in which many independent input variables impact a dependent output variable. The output variable’s name is the response. The RSM assesses all process factors concurrently while projecting an outcome as an enhanced methodical approach to experimentation [7]. The central composite design is one of the most crucial features of the RSM. Axial and factorial design points are included in the experiments’ three levels of the basic composite design. One of its key benefits is how quickly the ideal conditions may be identified with a few testing runs [8].
An algorithm that simulates biological neurons’ processing of data is called an artificial neural network (ANN). Most neural network models also include one or more hidden layers, varying in number depending on the type of research, in addition to input and output layers. The capacity of a neural network to perform computations internally to extract the required output from the input data is the most important feature. An ANN can be applied to complicated systems, since it is reliable and successful at simulating nonlinear interactions among factors and outcomes of diverse operations [9]. Based on mathematical computation, the ANFIS neural network was developed. Because it uses the Takagi–Sugeno fuzzy inference approach, it can deal with challenging nonlinear conditions. To provide precise and better predictions based on recorded input data, it mostly consists of a mixture of fuzzy algorithms and neural networks. The neural network regulates flexibility, while the fuzzy inference approach enhances the system’s reliability and dependability [1].
This work employed three-dimensional adsorbents from modified cellulose nanocrystals to adsorb zinc (II) from synthetic acid mine drainage. The novelty of the research originates from the modelling and analysis of zinc (II) adsorption capacity using artificial neural networks (ANNs), response surface methodology (RSM), and an adaptive neuro-fuzzy interference system (ANFIS), as well as the relationship between the output variable and four input variables, which include adsorption time, dosage, pH, and adsorbate concentration. The performance of the ANN, RSM, and ANFIS techniques is compared against statistically significant nonlinear error functions used to generate error distributions.
2. Materials and Methods
The CNCs utilised in this study were extracted from waste papers via acid hydrolysis (≥90%). Gelatine powder, hydrochloric acid (>99%), acetic acid (>98%), EDTA (>99%), hydrochloric acid (>99%), and sodium hydroxide (>99%) were purchased from Sigma-Aldrich. Gelatine and cellulose were combined to produce a three-dimensional composite of cellulose and gelatine successfully. A 50 mL cellulose nanocrystal suspension was added to 10 wt% gelatine after being fully dissolved in hot water (45 °C). As a cross-linking agent, 0.1 M EDTA was used. The unreacted compounds were subsequently removed from the three-dimensional composite using deionised water and preserved at 5 °C. Batch investigations were conducted in 250 mL glass vials with stoppers. Reactors with stoppered Erlenmeyer flasks carrying test solutions at room temperature (25 °C) with the appropriate Zn (II) concentration, contact time, pH, and adsorbent dose levels were used as input variables. For each test, 100 mL of a Zn (II) solution was added to the reactor. The pH of the solution was adjusted using diluted 0.1 M HCl or 0.1 M NaOH to maintain a constant pH throughout the experiment. Table 1 provides the range of process variables.

Table 1.
The range of variables used for the models.
3. Results and Discussion
For the experimental design, an analysis of variation, regression analysis, and optimisation of the process parameters for the removal of Zn (II), Design Expert version 13 was used. Figure 1a shows the interaction impact of the contact duration and dose at a pH of 6 and an unchanged concentration of 175 mg/L. As the contact duration increased, the dosage increased, and the adsorption capacity grew to 450 mg/g. This is because additional active adsorption sites were available for capturing Zn (II), and there was sufficient time for the adsorption process [9]. At a contact time and dose of 150 min and 150 mg/L, respectively, and the centre point of the plot, Figure 1b illustrates the combined effects of the pH and concentration. The contour plot appears as a straight line, indicating that the concentration and pH were interacting. As the pH increased within the specified range, the adsorption capacity decreased. The high concentration and lower pH enhanced the adsorption capability [9].
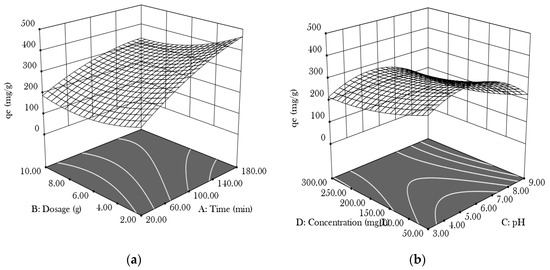
Figure 1.
The 3D surface plots of Zn (II) adsorption: (a) dosage and time; (b) concentration and pH.
The MATLAB software 2022a was used in determining the ANN and ANFIS. Using a multilayer perceptron, the network was trained (MPL). The neural network was trained using approximately 70% of the data sources, tested with 15%, and validated with the remaining 15% [9]. Levenberg–Marquardt, as shown in Figure 2, was the optimum technique for the training, testing, and validation. For the training, validation, testing, and all other phases combined, the value of R was 0.994, 0.998, 0.999, and 0.990.
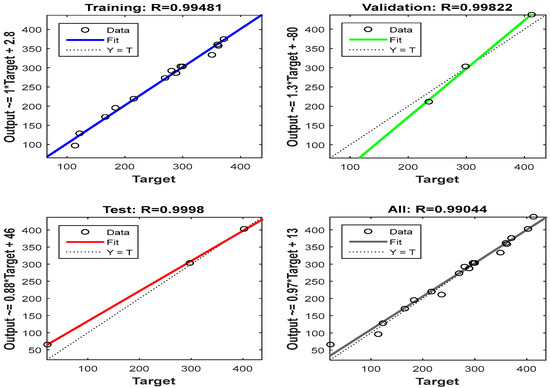
Figure 2.
Training, validation, and testing for the Levenberg–Marquardt algorithm.
The ANFIS has the benefit of being able to handle both numerical and language input variables in the adsorption results analysis. This makes it effective for simulating systems where certain input variables are challenging to quantify or when data are ambiguous [7]. The ANFIS can also provide insight into the connections between input and output variables, which can aid in understanding the underlying mechanics of the system under study [5] (Figure 3). Using modified cellulose nanocrystals, the fuzzy inference system network can predict the uptake of Zn (II) from the aqueous solution according to the high correlation value of 0.997 obtained from the ANFIS modelling. The fuzzy network proved suitable for simulating the removal of Zn (II) after seven training iterations with an error magnitude of 0.0005.
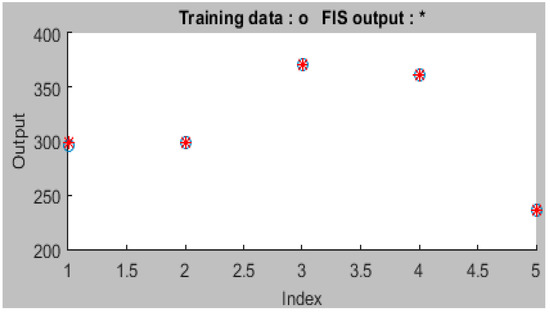
Figure 3.
Actual and predicted adsorption data for ANFIS.
To further assess the algorithm’s precision, two statistical error measures were used for the predictions generated with the model using the data in Table 2. Each model was assessed using the error functions RMSE and MSE. The statistical results show that the RSM and ANN models had the lowest accuracy in forecasting the Zn (II) adsorption process. The performance of the ANFIS model was marginally superior to the other two.

Table 2.
The RSM, ANN, and ANFIS statistical error analysis.
4. Conclusions
The effects of the process variables’ interactions and their ideal conditions were noted. A contact duration of 100 min, operating pH of 6, sorbent dose of 6 mg/L, Zn (II) concentration of 175 mg/L, and capacity for adsorption of 350.10 mg/g were the optimum conditions. Because of the employment of a tangential sigmoid transmission function at the hidden layer and a linear transfer function at the layer of the output, the Levenberg–Marquardt algorithm (four inputs, six hidden layers and one output) had the smallest MSE. The accuracy and similarity of the ANFIS, ANN, and RSM in forecasting Cr (VI) uptake were demonstrated. The ANFIS model, followed by the ANN and RSM, had the most exceptional quality and dependability based on two statistical error indices.
Author Contributions
Conceptualisation, M.B.; methodology, M.B. and T.S.; software, M.B.; validation, M.B. and T.S.; formal analysis, M.B. and T.S.; investigation, M.B. and T.S.; resources, M.B. and T.S.; data curation, T.S.; writing—original draft preparation, M.B.; writing—review and editing, M.B. and T.S.; visualisation, M.B. and T.S.; project administration, T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The author would like to thank the Vaal University of Technology’s Department of Chemical & Metallurgical Engineering for providing the continuous operating facilities.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| RMSE | Root mean square error |
| BP | Back propagation |
| LM | Levenberg–Marquardt model |
| CNCs | Cellulose nanocrystals |
| ANFIS | Adaptive neuro-fuzzy inference system |
References
- Azad, H.; Mohsennia, M.; Cheng, C.; Amini, A. Facile fabrication of PVB-PVA blend polymer nanocomposite for simultaneous removal of heavy metal ions from aqueous solutions: Kinetic, equilibrium, reusability and adsorption mechanism. J. Environ. Chem. Eng. 2021, 9, 106214. [Google Scholar] [CrossRef]
- Kabuba, J.; Banza, M. Results in Engineering Ion-exchange process for the removal of Ni (II) and Co (II) from wastewater using modified clinoptilolite: Modeling by response surface methodology and artificial neural network. Results Eng. 2020, 8, 100189. [Google Scholar] [CrossRef]
- Kabuba, J.; Banza, M. Modification of clinoptilolite with dialkylphosphinic acid for the selective removal of cobalt (II) and nickel (II) from hydrometallurgical effluent. Can. J. Chem. Eng. 2021, 99, S168–S178. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]
- Banza, M.; Rutto, H. Selective removal of Cr (VI) from hydrometallurgical effluent using modified cellulose nanocrystals (CNCs) with succinic anhydride and ethylenediaminetetraacetic acid: Isotherm, kinetics, and thermodynamic studies. Can. J. Chem. Eng. 2023, 101, 896–908. [Google Scholar] [CrossRef]
- Danial, W.H.; Majid, Z.A.; Muhid, M.N.M.; Triwahyono, S.; Bakar, M.B.; Ramli, Z. The reuse of wastepaper for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2015, 118, 165–169. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nano fi brils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.M.; Horsfall, I.T.; Onuoha-Ukoha, E.; Osa-aria, K. Application of hybrid ANFIS-based non-linear regression modeling to predict the %oil yield from grape peels: Effect of process parameters and FIS generation techniques. Clean. Eng. Technol. 2022, 6, 100371. [Google Scholar] [CrossRef]
- Banza, M.; Rutto, H.; Seodigeng, T. Soil and Sediment Contamination: An International Application of Artificial Neural Network and Shrinking Core Model for Copper (II) and Lead (II) Leaching from Contaminated Soil Using Ethylenediaminetetraacetic Acid Application of Artificial Neural N. Soil Sediment Contam. Int. J. 2023, 1–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).