Abstract
I filled the single-walled carbon nanotubes (SWCNTs) with gallium selenide (GaSe) and rubidium iodide (RbI) as p- and n-dopant chemical compounds. The filling was confirmed by high-resolution transmission electron microscopy. I investigated the electronic properties by Raman spectroscopy, optical absorption spectroscopy, near-edge X-ray absorption fine structure spectroscopy, and X-ray photoelectron spectroscopy. I proved the p-doping of SWCNTs by the introduced GaSe. The data featured the n-doping of carbon nanotubes in RbI-filled SWCNTs.
1. Introduction
The interest in single-walled carbon nanotubes led to many interesting applications, such as nanoelectronics, thermoelectric power generation devices, optoelectronics, biomedicine, sensors, catalysis, and electrochemical energy storage devices. The filled SWCNTs are promising components of these devices. An approach to tailoring the properties of SWCNTs by filling was started in our work. After that, the SWCNTs were filled with chalcogenides and halogenides of metals, and the modified properties were studied by Raman spectroscopy, optical absorption spectroscopy, X-ray absorption spectroscopy, and photoemission spectroscopy [1,2,3]. It was shown that electron donors and electron acceptors modify the properties of SWCNTs. Modifications of spectra are changes in peak positions, profiles of bands, and the appearance of new peaks [4,5,6]. The methods of filling SWCNTs were developed to fill SWCNTs in large ratios. Among them are liquid-phase methods of filling with metals, melt methods of filling with metal halogenides, and gas methods of filling with molecules. It is difficult to fill metal chalcogenides inside SWCNTs in a single step process because they have large melting points. In this work, I fill the SWCNTs with gallium selenide by a single-step process. The melting point of GaSe is 960 °C, and this is a unique case of the filling of a high-melting compound in SWCNTs by the melt method. I investigate the electronic properties of filled SWCNTs using spectroscopic techniques to study the influence of encapsulated compounds on SWCNTs.
The filler inside SWCNTs leads to an electron donor or electron acceptor doping effect. There are many compounds that are encapsulated inside SWCNTs. Among them are metal halogenides, metal chalcogenides, and metals. The influence of substances on the electronic properties of SWCNTs is determined by their work function (WF). There are three cases. If the work function of a substance is larger than the WF of SWCNTs, the p-doping of carbon nanotubes is observed. If the work function of a substance is smaller than the WF of SWCNTs, n-doping of SWCNTs occurs. If the work function of a substance equals the WF of SWCNTs, no modification of the electronic properties is observed. Typical donors of electrons are metals with a WF that is significantly smaller than the WF of SWCNTs. Molecules can also lead to donor doping of SWCNTs. Among metal halogenides, rubidium iodide has a smaller WF than the value of SWCNTs. It is expected to have a n-doping effect on SWCNTs. The filling of SWCNTs with rubidium iodide is a convenient way of n-doping SWCNTs. This is caused by several reasons. Firstly, the filling of SWCNTs with RbI is made by the single-step melt method, which is simple and leads to clean samples. Secondly, RbI has a low melting point, which simplifies the combinations of synthesis and integration processes at the lab and industrial scales. Thirdly, RbI has a very low WF (about 1 eV lower than the value of SWCNTs). This can result in very large modifications of the Fermi level of SWCNTs. The downshift of several hundreds of meV is expected.
2. Experimental
I put the SWCNTs and GaSe in a quartz ampoule, sealed it under vacuum, and heated it to a temperature of 1060 °C, which is higher by 100 °C than the melting point of GaSe. The SWCNTs were pre-opened by annealing in air at 500 °C for 30 min. The diameter of SWCNTs is 1.4 nm. The quartz ampoule was cooled down to room temperature to crystallize the compound inside the SWCNTs. RbI was placed inside the quartz ampoule where SWCNTs were located. The system was maintained at a temperature that is 100 °C higher than the melting point of RbI (Tmelting(RbI) = 656 °C). The samples were cooled slowly to room temperature. The electronic properties of SWCNTs filled with GaSe and RbI were investigated by Raman spectroscopy, optical absorption spectroscopy, near-edge X-ray absorption fine structure spectroscopy (NEXAFS), and photoemission spectroscopy (X-ray photoelectron spectroscopy, XPS).
3. Results
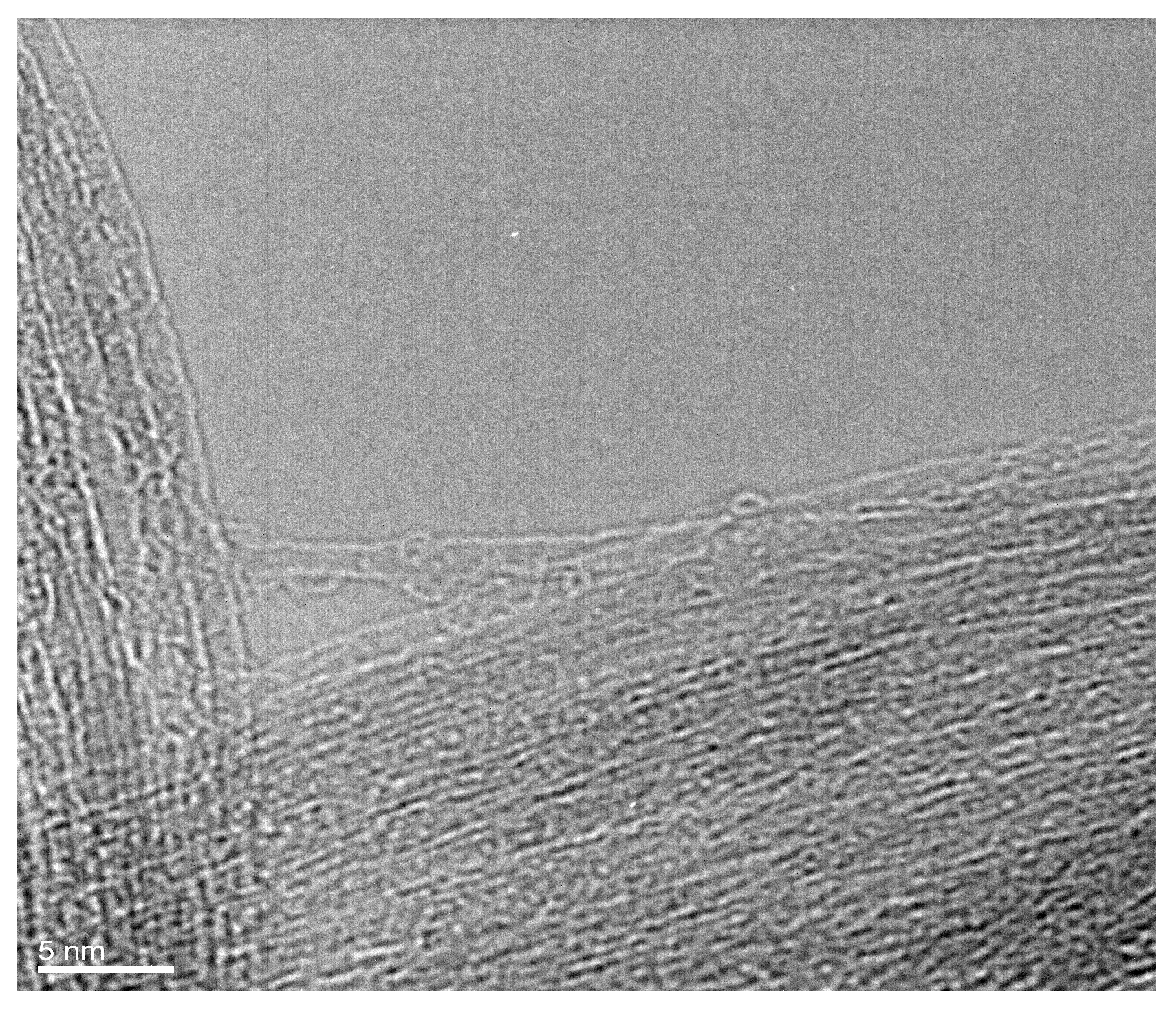
It was found that GaSe is filled inside SWCNTs. Figure 1 shows the high-resolution transmission electron microscopy (HRTEM) image of a GaSe-filled SWCNT bundle. It is visible that the channels of carbon nanotubes are filled. The homogenous filling of carbon nanotubes is confirmed. The filling materials are recognized inside the inner cavities of carbon nanotubes.
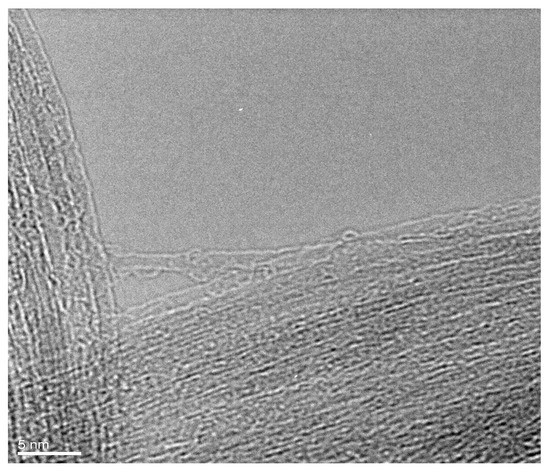
Figure 1.
The HRTEM image of a bundle of GaSe-filled SWCNTs.
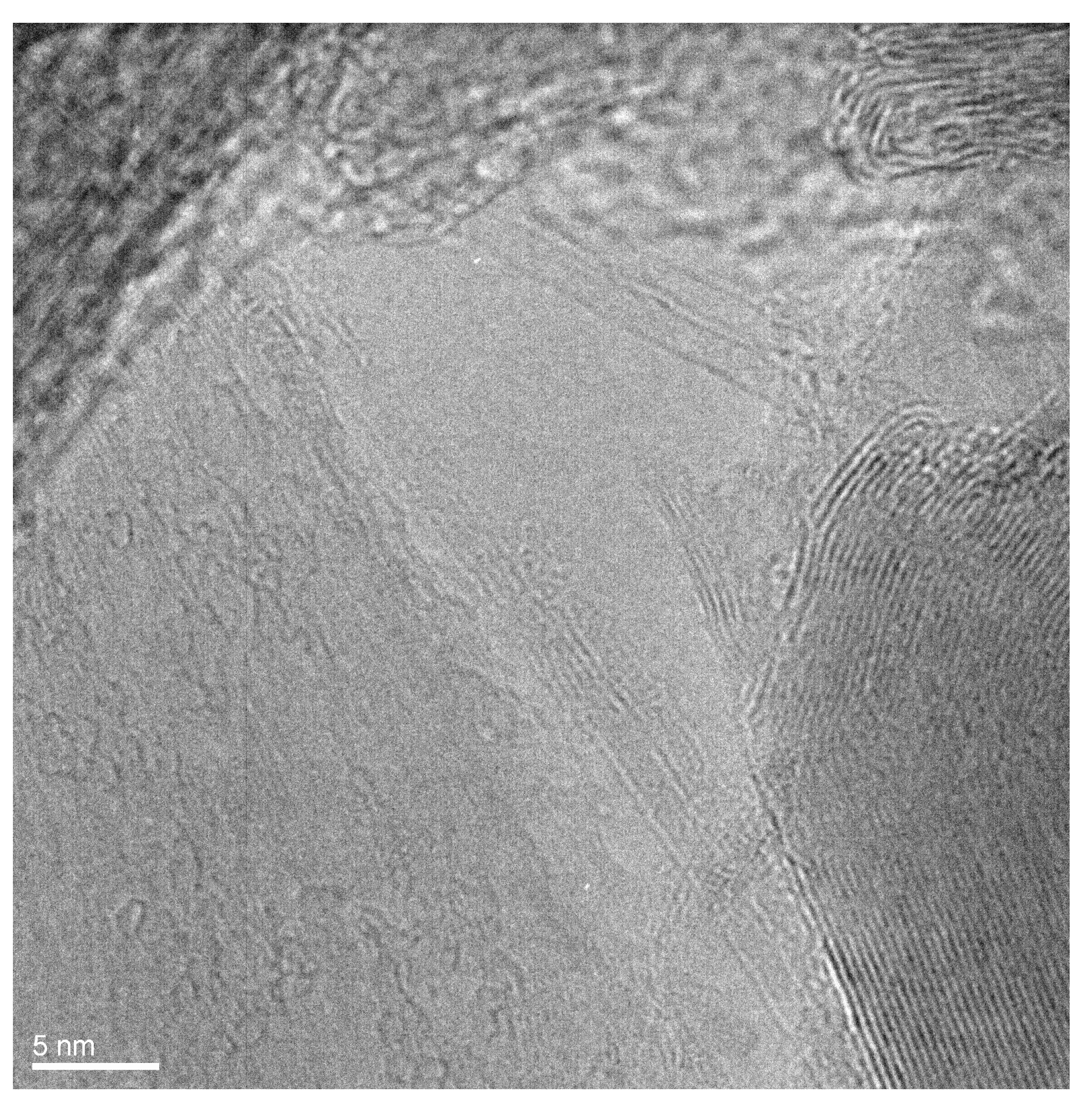
Figure 2 presents the HRTEM image of individual filled SWCNTs. In this image, one can see the two filled SWCNTs at the bottom. It is visible that there is a filler within the walls of carbon nanotubes. For comparison, the two unfilled SWCNTs are visible in the middle of the image. It is visible that the space inside the channels is not filled. Thus, it is obvious that GaSe is filled inside SWCNTs.
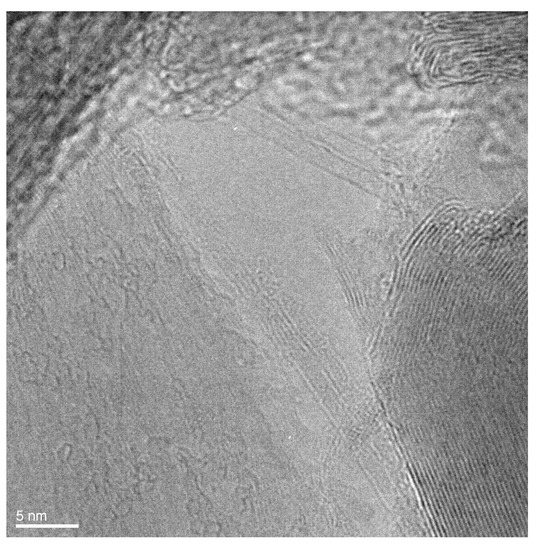
Figure 2.
The HRTEM image of individual GaSe-filled SWCNTs.
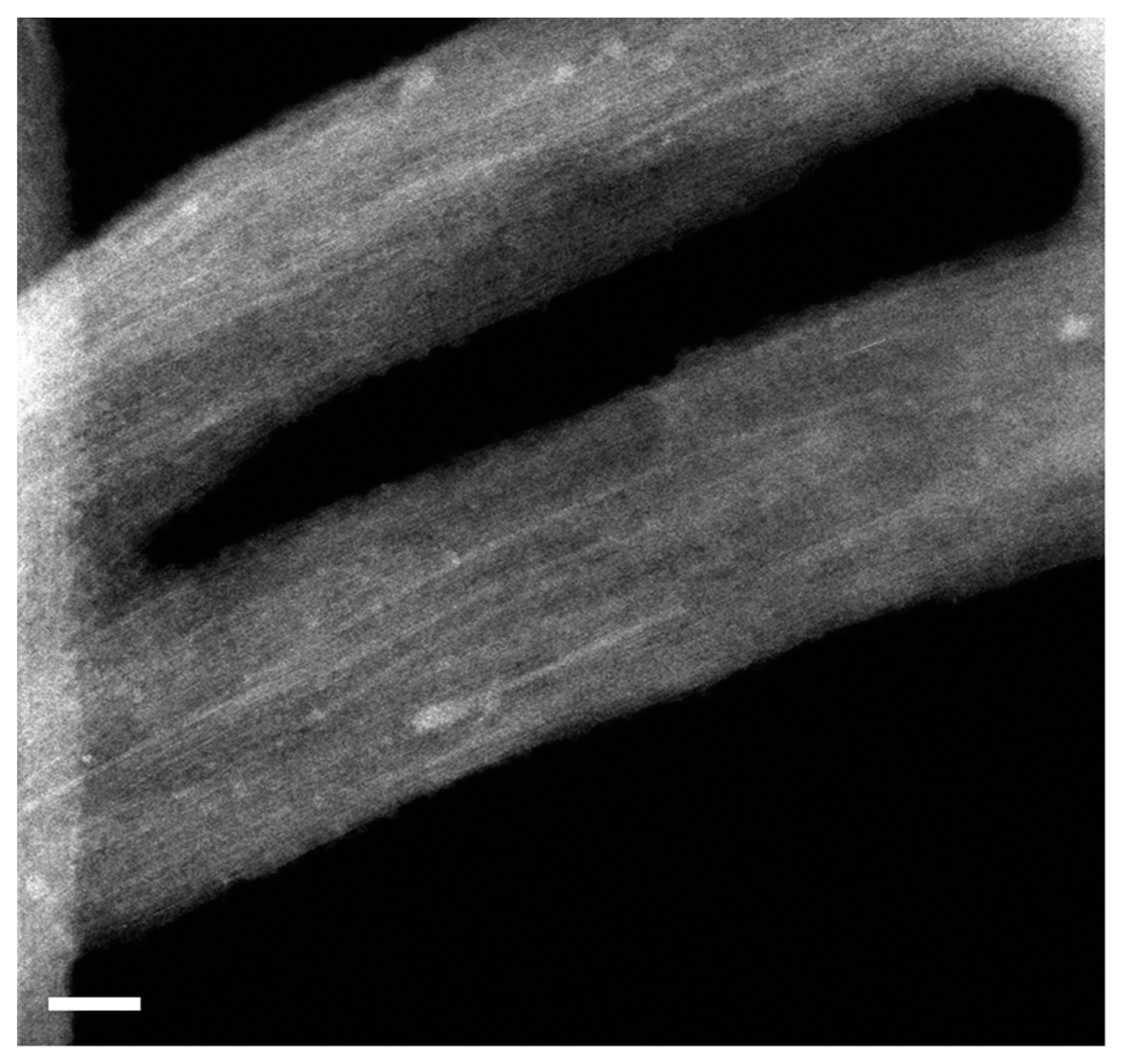
Here, I show the HRTEM data of RbI-filled SWCNTs. Figure 3 shows the low-magnification image of the filled SWCNTs. It is visible that there are encapsulated materials in channels of carbon nanotubes. They are filled throughout the sample. White contrast lines correspond to filler within SWCNTs.
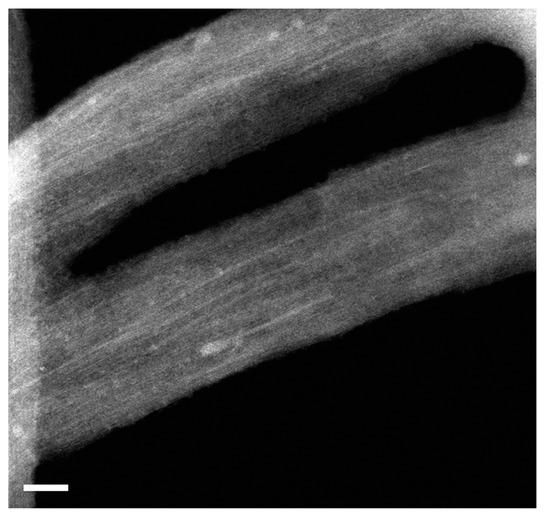
Figure 3.
The HRTEM image of bundles of RbI-filled SWCNTs. The scale bar is 10 nm.
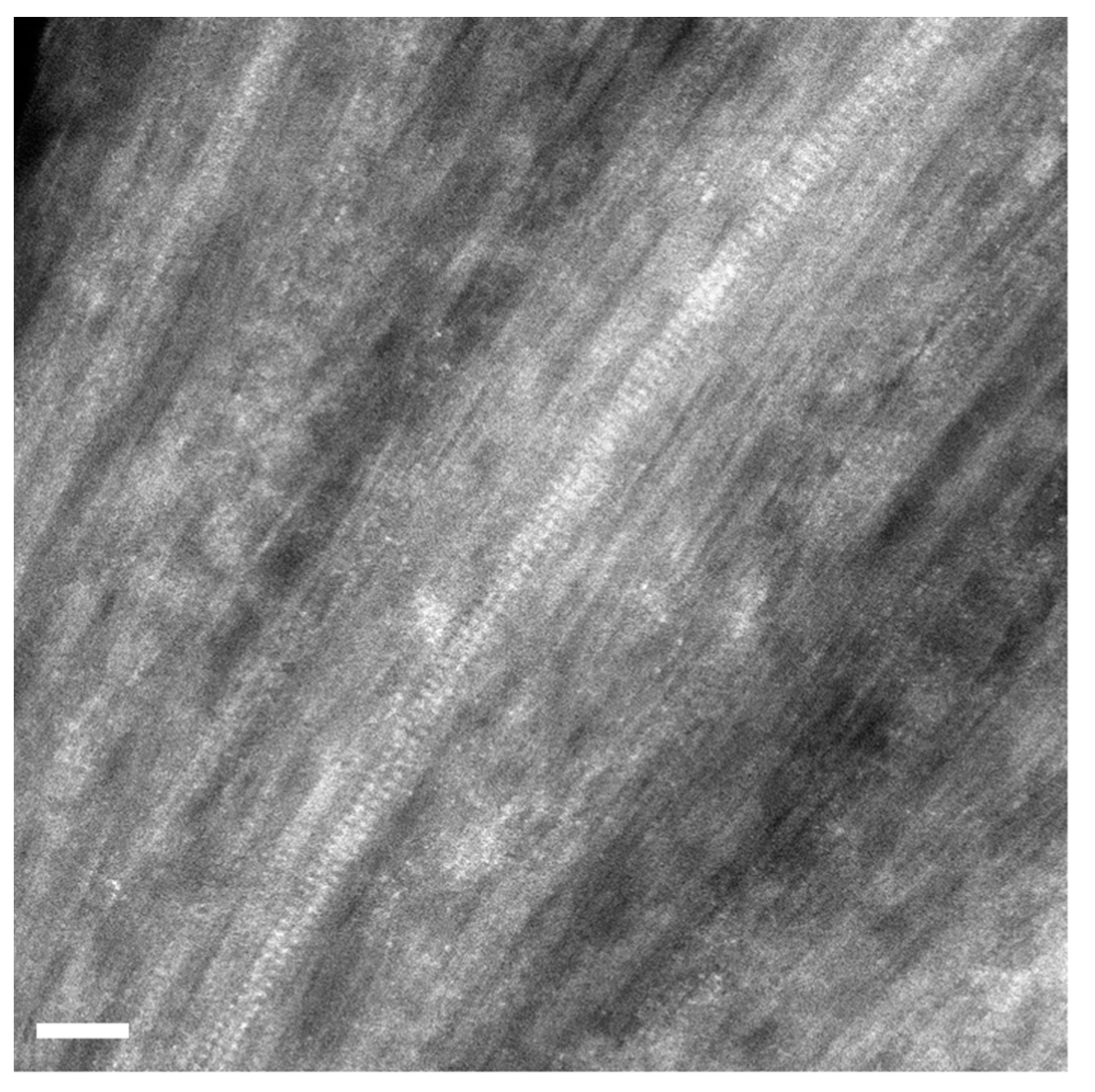
Figure 4 presents a high-magnification image of filled SWCNTs. The atoms of the introduced salt are visible inside carbon nanotubes. The atoms are white dots. They are located in two columns, and atoms in columns are periodically positioned. There are pairs of atoms that are symmetrically located within the SWCNTs. The walls of SWCNTs are also visible, and it is obvious that the diameter of atomically thick crystals is smaller than the diameter of SWCNTs. The crystals inside SWCNTs have a cubic structure, as in the case of bulk compounds [7].
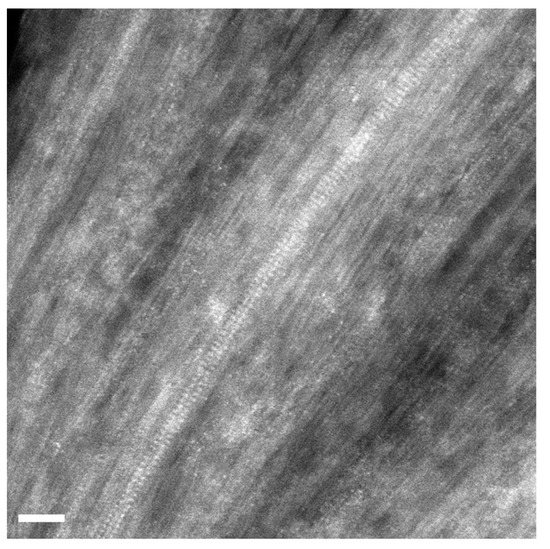
Figure 4.
The HRTEM image of individual RbI-filled SWCNTs. The scale bar is 2 nm.
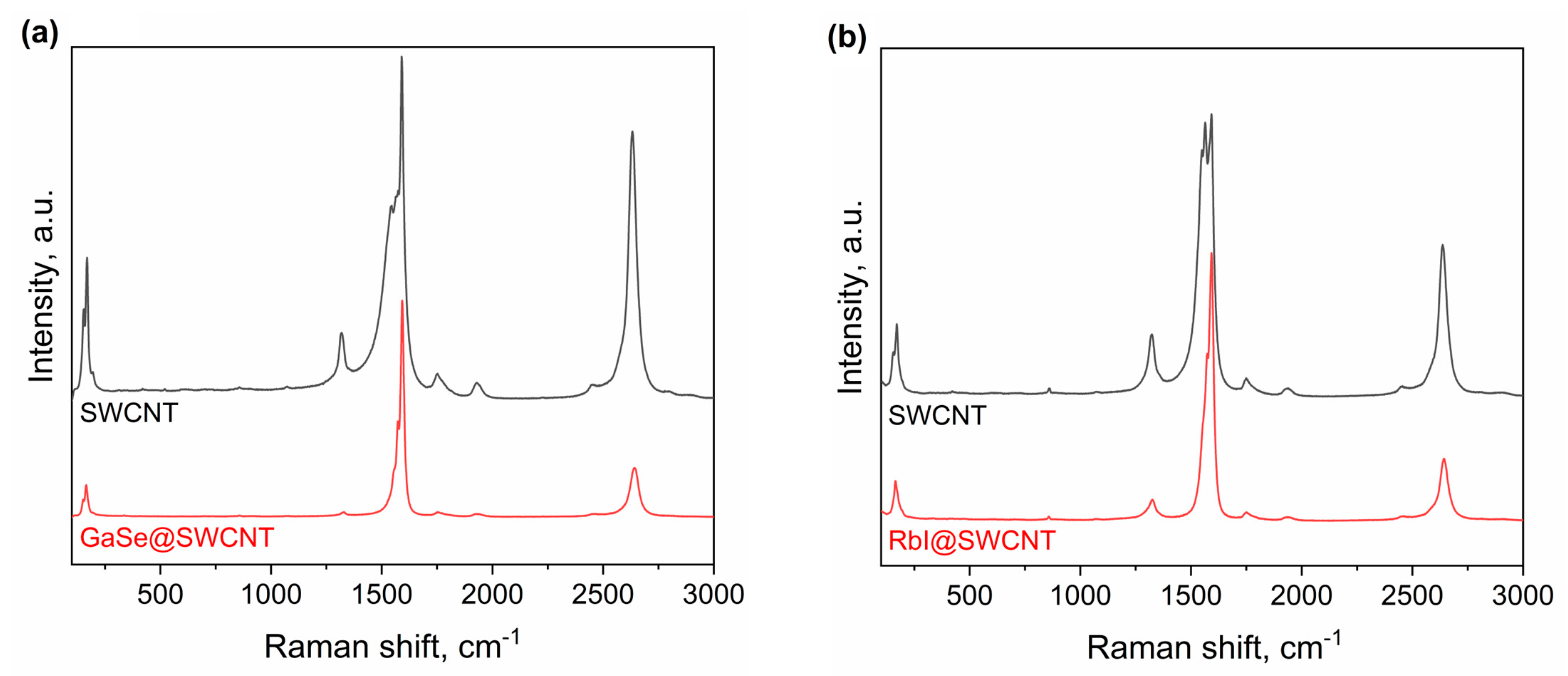
The data from Raman spectroscopy shows that GaSe has an electron acceptor effect on SWCNTs (Figure 5a). The NEXAFS shows no formation of chemical bonds between SWCNTs and GaSe. The data of XPS shows the shift by 0.28 eV to lower binding energies. This testifies to the p-doping of SWCNTs by the introduced compound. The electronic properties of SWCNTs filled with RbI were investigated by Raman spectroscopy and X-ray photoelectron spectroscopy. It was shown that RbI has a n-doping effect on SWCNTs (Figure 5b). This is a unique case of n-doping of SWCNTs by metal halogenide, and the success of this experiment opens new ways to applications of filled SWCNTs in nanoelectronics, including p-n transitions with similar morphology and atomic structure compounds, and simple preparation methods, integration routes, cleaning procedures, and manipulation in devices. This filling is a very important achievement, which opens a new chapter in the applications of filled SWCNTs because it gives a lot of information on chemical and physical properties.
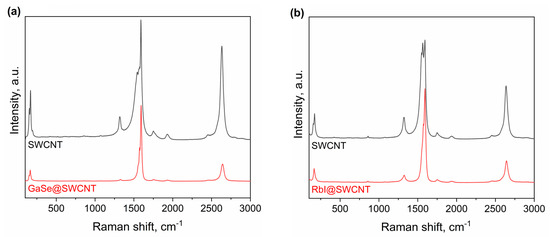
Figure 5.
The Raman spectra of GaSe-filled SWCNTs (a) and RbI-filled SWCNTs acquired at laser wavelength of 633 nm (b) in comparison with the spectra of the pristine SWCNTs.
4. Conclusions
In this work, I filled SWCNTs with GaSe and investigated the electronic properties using spectroscopic techniques. The HRTEM data proved the filling of SWCNTs, and the data of four spectroscopic methods confirmed the p-doping of SWCNTs by encapsulated GaSe. I filled SWCNTs with RbI using the melt method. I investigated the atomic structure of filled SWCNTs by HRTEM. It was shown that the channels of SWCNTs are filled. The electronic properties of filled SWCNTs were investigated by Raman spectroscopy and X-ray photoelectron spectroscopy. The introduced compound leads to the n-doping of SWCNTs. This leads to easy preparation, integration, and application of RbI-filled devices.
Funding
These studies were partly performed during the implementation of the project Building-up Center for advanced materials application of the Slovak Academy of Sciences, ITMS project code 313021T081 supported by the Research and Innovation Operational Program funded by the ERDF.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kashtiban, R.J.; Burdanova, M.G.; Vasylenko, A.; Wynn, J.; Medeiros, P.V.C.; Ramasse, Q.; Morris, A.J.; Quigley, D.; Lloyd-Hughes, J.; Sloan, J. Linear and Helical Cesium Iodide Atomic Chains in Ultranarrow Single-Walled Carbon Nanotubes: Impact on Optical Properties. ACS Nano 2021, 15, 13389–13398. [Google Scholar] [CrossRef] [PubMed]
- Vorfolomeeva, A.A.; Stolyarova, S.G.; Asanov, I.P.; Shlyakhova, E.V.; Plyusnin, P.E.; Maksimovskiy, E.A.; Gerasimov, E.Y.; Chuvilin, A.L.; Okotrub, A.V.; Bulusheva, L.G. Single-Walled Carbon Nanotubes with Red Phosphorus in Lithium-Ion Batteries: Effect of Surface and Encapsulated Phosphorus. Nanomaterials 2023, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Vorfolomeeva, A.A.; Pushkarevsky, N.A.; Koroteev, V.O.; Surovtsev, N.V.; Chuvilin, A.L.; Shlyakhova, E.V.; Plyusnin, P.E.; Makarova, A.A.; Okotrub, A.V.; Bulusheva, L.G. Doping of Carbon Nanotubes with Encapsulated Phosphorus Chains. Inorg. Chem. 2022, 61, 9605–9614. [Google Scholar] [CrossRef] [PubMed]
- Fedoseeva, Y.V.; Bulusheva, L.G.; Okotrub, A.V.; Vyalikh, D.V.; Fonseca, A. XANES Investigation of Pristine and Fluorinated Single-Walled Carbon Nanotubes Before and After Annealing. Fuller. Nanotub. Carbon Nanostructures 2010, 18, 595–599. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Orekhov, A.S.; Chekhova, G.N.; Koroteev, V.O.; Kanygin, M.A.; Senkovskiy, B.V.; Chuvilin, A.; Pontiroli, D.; Riccò, M.; Bulusheva, L.G.; et al. Single-Walled Carbon Nanotube Reactor for Redox Transformation of Mercury Dichloride. ACS Nano 2017, 11, 8643–8649. [Google Scholar]
- Sedelnikova, O.V.; Gurova, O.A.; Makarova, A.A.; Fedorenko, A.D.; Nikolenko, A.D.; Plyusnin, P.E.; Arenal, R.; Bulusheva, L.G.; Okotrub, A.V. Light-Induced Sulfur Transport inside Single-Walled Carbon Nanotubes. Nanomaterials 2020, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Overton, T.; Rourke, J.; Armstrong, F. Inorganic Chemistry, 6th ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).