Field Trial of Solar-Powered Ion-Exchange Resin for the Industrial Wastewater Treatment Process †
Abstract
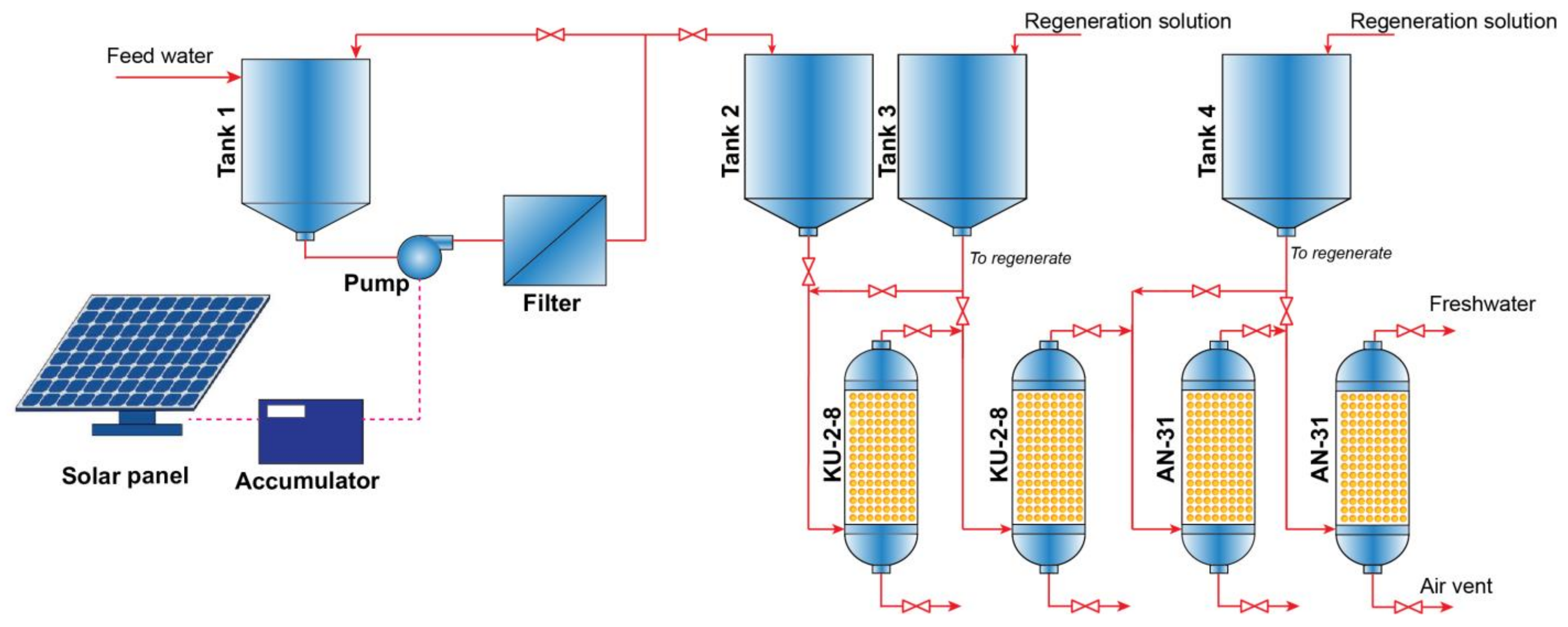
:1. Introduction
2. Materials and Methods

3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TDS | Total dissolved solids |
| MSF | Multi-stage-flash distillation |
| MED | Multi-effect-distillation |
| RO | Reverse osmosis |
| FO | Forward osmosis |
| ED | Electro dialysis |
| EDR | Electro dialysis-reverse |
| H/DH | Humidification/dehumidification |
| MD | Membrane distillation |
| SSD | Solar still distillation |
| CDI | Capacitive deionization |
| IXR | Ion-exchange resin |
References
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse Osmosis Desalination: A State-of-the-Art Review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Darre, N.C.; Toor, G.S. Desalination of water: A Review. Curr. Pollut. Rep. 2018, 4, 104–111. [Google Scholar] [CrossRef]
- Esrafilian, M.; Ahmadi, R. Energy, Environmental and Economic Assessment of a Polygeneration System of Local Desalination and CCHP. Desalination 2019, 454, 20–37. [Google Scholar] [CrossRef]
- Nair, M.; Kumar, D. Water Desalination and Challenges: The Middle East Perspective: A Review. Desalin. Water Treat 2013, 51, 2030–2040. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Atieh, M.A.; Sajid, M.; Nazal, M.K. Desalination and Environment: A Critical Analysis of Impacts, Mitigation Strategies, and Greener Desalination Technologies. Sci. Total Environ. 2021, 780, 146585. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Ramadan, M.; Olabi, A.G. Environmental Impact of Emerging Desalination Technologies: A Preliminary Evaluation. J. Environ. Chem. Eng. 2020, 8, 104099. [Google Scholar] [CrossRef]
- Panagopoulos, A. Water-Energy Nexus: Desalination Technologies and Renewable Energy Sources. Environ. Sci. Pollut. Res. 2021, 28, 21009–21022. [Google Scholar] [CrossRef] [PubMed]
- Curto, D.; Franzitta, V.; Guercio, A. A Review of the Water Desalination Technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Kamolov, A.; Turakulov, Z.; Rejabov, S.; Díaz-Sainz, G.; Gómez-Coma, L.; Norkobilov, A.; Fallanza, M.; Irabien, A. Decarbonization of Power and Industrial Sectors: The Role of Membrane Processes. Membranes 2023, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Saleh, L.; Mezher, T. Techno-Economic Analysis of Sustainability and Externality Costs of Water Desalination Production. Renew. Sustain. Energy Rev. 2021, 150, 111465. [Google Scholar] [CrossRef]
- Davies, P.A.; Afifi, A.; Khatoon, F.; Kuldip, G.; Javed, S.; Khan, S.J. Double-Acting Batch-RO System for Desalination of Brackish Water with High Efficiency and High Recovery. Desalin. Water Treat 2021, 224, 1–11. [Google Scholar] [CrossRef]
- Pearce, G.K. UF/MF Pre-Treatment to RO in Seawater and Wastewater Reuse Applications: A Comparison of Energy Costs. Desalination 2008, 222, 66–73. [Google Scholar] [CrossRef]
| Name of Pointers | Unit | Plant’s Requirement for Treatment of Wastewater (No More Than) | Results of Analysis of Mixed Wastewater before Treatment in Equipment | Results of Analysis of Mixed Wastewater after Treatment in Equipment |
|---|---|---|---|---|
| Total hardness | mEq/L | 3.0 | 9.3 | 0.27 |
| Total alkalinity | - | 0.6 | 3.6 | 0.52 |
| Calcium (Ca2+) | mEq/L | 3.0 | 4.4 | 0.27 |
| Magnesium (Mg2+) | mEq/L | - | 4.9 | - |
| Chlorides (Cl−) | mg/L | 366.15 | 553 | 16.5 |
| Sulfates (SO42−) | mg/L | 662.81 | 827 | 10.6 |
| Total dissolved solids (TDS) | mg/L | 1605.81 | 1885.0 | 27.3 |
| Suspended solids | mg/L | 1.5 | 1.014 | 0.042 |
| Hydrogen carbonate indicator (pH) | - | 7/7.5 | 9.2 | 7.5 |
| Technology | Input | Recovery Ratio | Water Quality [ppm] | Energy Consumption Electrical [kWh/m3] | Energy Consumption Thermal [kWh/m3] |
|---|---|---|---|---|---|
| MSF | Brackish water | 0.33 | 10 | 2.5–4 | 7.5–12 |
| MED | Brackish water | 0.34 | 10 | 1.5–2 | 4–7 |
| RO | Wastewater | 0.65 | 200–500 | 1.5–2.5 | - |
| IXR | Wastewater | 0.8 | 27.3 | 1.2–1.8 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshbobaev, J.; Norkobilov, A.; Turakulov, Z.; Khamidov, B.; Kodirov, O. Field Trial of Solar-Powered Ion-Exchange Resin for the Industrial Wastewater Treatment Process. Eng. Proc. 2023, 37, 47. https://doi.org/10.3390/ECP2023-14626
Eshbobaev J, Norkobilov A, Turakulov Z, Khamidov B, Kodirov O. Field Trial of Solar-Powered Ion-Exchange Resin for the Industrial Wastewater Treatment Process. Engineering Proceedings. 2023; 37(1):47. https://doi.org/10.3390/ECP2023-14626
Chicago/Turabian StyleEshbobaev, Jaloliddin, Adham Norkobilov, Zafar Turakulov, Bakhodir Khamidov, and Orifjon Kodirov. 2023. "Field Trial of Solar-Powered Ion-Exchange Resin for the Industrial Wastewater Treatment Process" Engineering Proceedings 37, no. 1: 47. https://doi.org/10.3390/ECP2023-14626
APA StyleEshbobaev, J., Norkobilov, A., Turakulov, Z., Khamidov, B., & Kodirov, O. (2023). Field Trial of Solar-Powered Ion-Exchange Resin for the Industrial Wastewater Treatment Process. Engineering Proceedings, 37(1), 47. https://doi.org/10.3390/ECP2023-14626







