Abstract
Here, I fill large 1.7 nm diameter single-walled carbon nanotubes (SWCNTs) with silver chloride (AgCl). I present photoemission insights into the filling of SWCNTs. C1s X-ray photoelectron spectroscopy (XPS) reveals the p-doping of SWCNTs. The Raman spectroscopy data are complementary to the XPS data, and they confirm the strong doping effect of encapsulated silver chloride on SWCNTs.
1. Introduction
Single-walled carbon nanotubes (SWCNTs) represent a container that can be filled with different substances for various applications. Silver chloride has long been used as a photoactive chemical compound that can be applied in biomedicine. SWCNTs prevent filler from destruction, and the encapsulated substance can at the same time modify the properties of carbon nanotubes. There are two methods for investigating the electronic properties of filled carbon nanotubes: X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy. X-ray photoelectron spectroscopy is a viable tool for the characterization of carbon nanotubes [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. It is a non-destructive, useful method to reveal the Fermi level shift in filled SWCNTs. The shifts in the peaks can be directly attributed to the Fermi level shifts and work function variations [20]. In our previous work, we studied small-diameter metallic AgCl-filled SWCNTs [21] and semiconducting AgCl-filled SWCNTs [22]. We observed all differences in varieties of SWCNTs filled with strong filler. The influence of filler on single chiralities with a metallic and semiconducting character was investigated. In this study, I filled large 1.7 nm diameter SWCNTs with AgCl to make photoemission insights into the filling of SWCNTs. My motivation is the following. Firstly, the filling of large-diameter SWCNTs allows for the encapsulation of a greater amount of material inside carbon nanotubes. This leads to a larger doping effect of filler on SWCNTs. Secondly, these materials were not investigated by photoemission spectroscopy nor Raman spectroscopy to reveal doping effects. Thirdly, 1.7 nm diameter SWCNTs are prepared via a simple chemical vapour deposition (CVD) method which allows the filling of large amounts of pure SWCNTs with the proposed method, and it opens the way to industrial-scale preparation and applications.
2. Experimental Section
I filled SWCNTs with AgCl using the following method. I put SWCNTs and AgCl in a quartz ampoule, pumped them under vacuum, and sealed them. The ampoule was heated to a temperature that was 100 °C higher than melting point of AgCl (Tmelting(AgCl) = 455 °C). The system was maintained at this temperature for 6 h, and then cooled. The electronic properties of filled SWCNTs were investigated using XPS and Raman spectroscopy.
3. Results
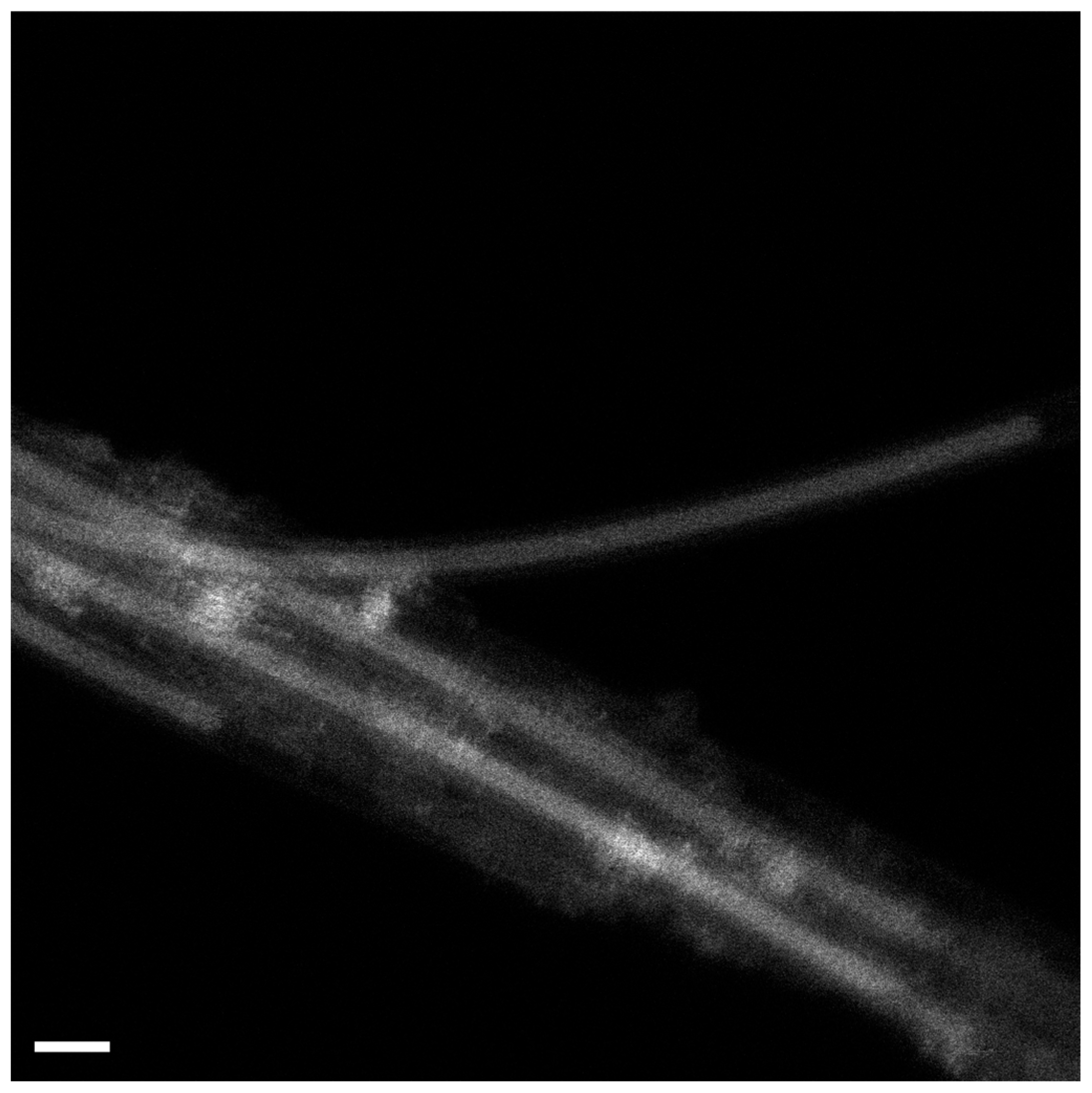
Here, Figure 1 shows an example of a high-resolution transmission electron microscopy (HRTEM) image of AgCl-filled SWCNTs. It is visible that the channels of SWCNTs are filled. In the image, one can observe three individual carbon nanotubes with AgCl inside the channels. The structure of the crystal can be seen. The structure of bulk AgCl resembles a NaCl structure Fm3m space group (a = 0.546 nm) [23].
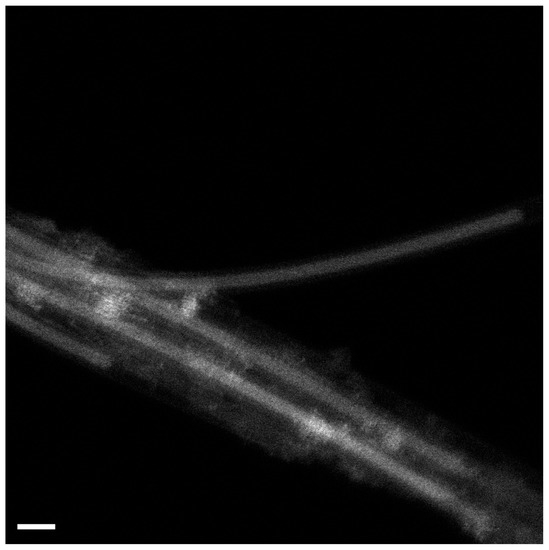
Figure 1.
The HRTEM data of AgCl-filled SWCNTs.
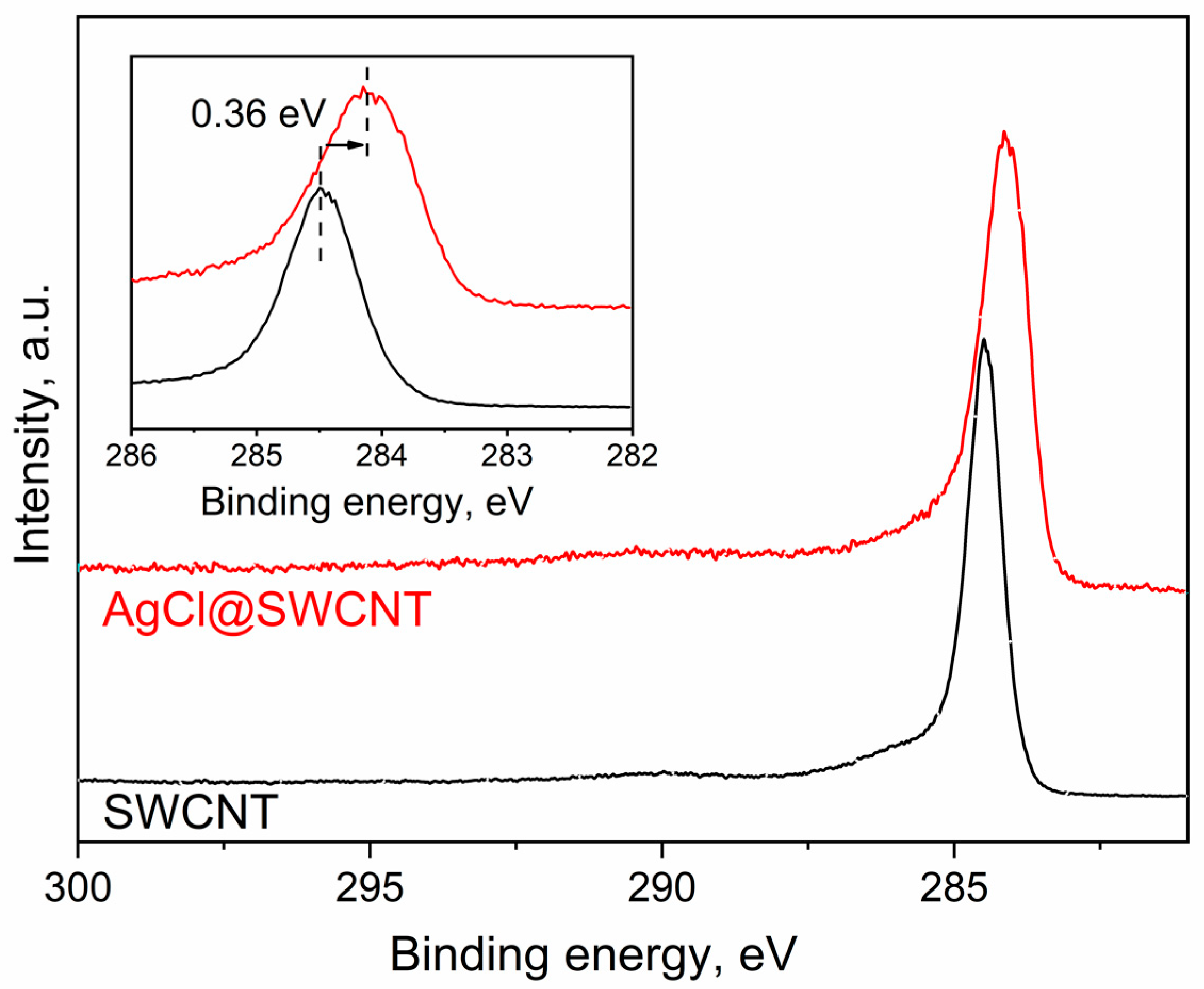
In Figure 2, I show the C 1s XPS spectra of pristine SWCNTs, and AgCl-filled carbon nanotubes. The C 1s XPS spectra represent the single peaks. The peak of AgCl-filled SWCNTs is shifted by 0.36 eV to lower binding energies compared to the spectrum of the pristine SWCNTs. The spectrum of the filled SWCNTs is broadened in comparison with that of the pristine SWCNTs. These changes confirm the change in the electronic properties of SWCNTs upon filling, and they reveal the p-doping of SWCNTs.
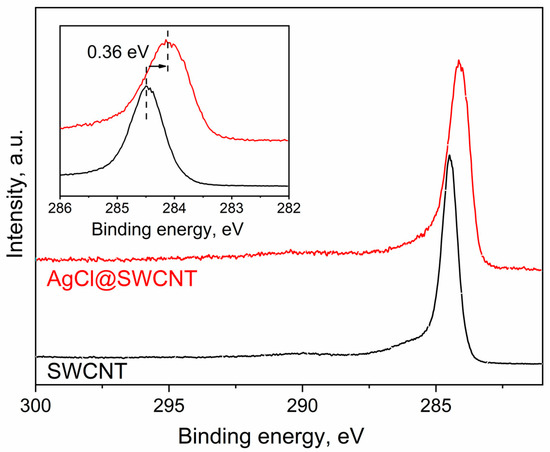
Figure 2.
The C 1s XPS spectra of the pristine 1.7 nm diameter SWCNTs and AgCl-filled SWCNTs.
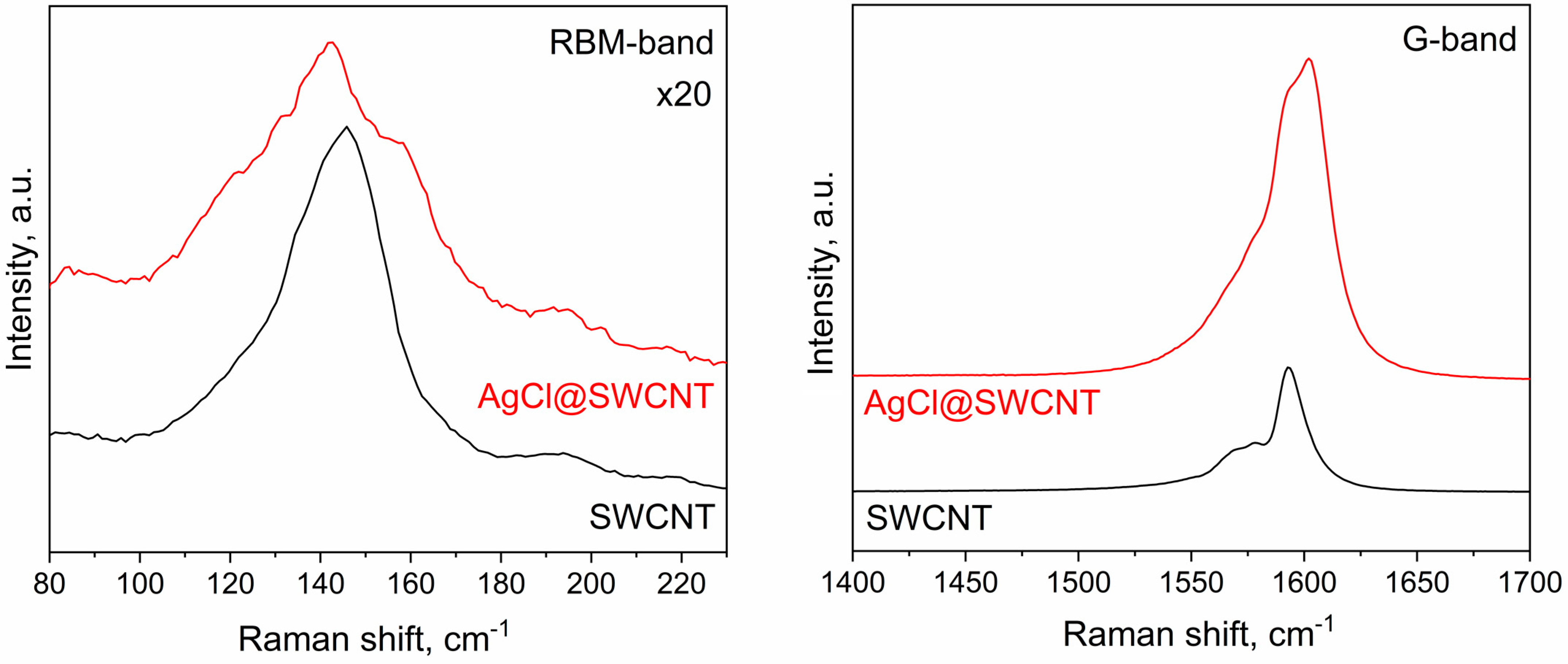
In Figure 3, I show the Raman spectroscopy data of AgCl-filled SWCNTs in comparison with the data of the pristine carbon nanotubes. These data are complementary to the photoemission spectroscopy data. The Raman spectrum of filled SWCNTs has differences in radial breathing mode (RBM) and the G-band. In the RBM-band, there are modifications in the band profile which were caused by alterations in the peak intensities. In the G-band, there are shifts in the peaks. The Breit–Wigner–Fano GBWF, peak of tangential contribution GTO, and peak of longitudinal contribution GLO are shifted to higher frequencies, leading to a shift in the whole spectrum by about 10 cm−1. This corresponds to the p-doping of SWCNTs by AgCl, and these results confirm the photoemission spectroscopy data.
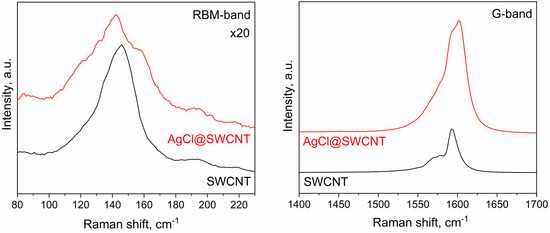
Figure 3.
The RBM and G-bands of Raman spectra of the pristine 1.7 nm diameter SWCNTs and AgCl-filled SWCNTs.
4. Conclusions
In this work, I filled SWCNTs with AgCl, and I made photoemission insights into the filling of SWCNTs with AgCl. It was revealed that AgCl has a p-doping effect on SWCNTs. The proposed filling method allows for filling SWCNTs synthesized by an industrial CVD method simply and quickly. It opens up possibilities for industrial applications of filled SWCNTs.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I thank Andreas Mittelberger (University of Vienna, Vienna, Austria) for the HRTEM measurements, and Oleg Domanov (University of Vienna, Vienna, Austria) for the XPS measurements.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kharlamova, M.V. Kinetics, Electronic Properties of Filled Carbon Nanotubes Investigated with Spectroscopy for Applications. Nanomaterials 2022, 13, 176. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Metallocene-Filled Single-Walled Carbon Nanotube Hybrids. Nanomaterials 2023, 13, 774. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Applications of Filled Single-Walled Carbon Nanotubes: Progress, Challenges, and Perspectives. Nanomaterials 2021, 11, 2863. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Electrochemistry of Carbon Materials: Progress in Raman Spectroscopy, Optical Absorption Spectroscopy, and Applications. Nanomaterials 2023, 13, 640. [Google Scholar] [CrossRef] [PubMed]
- Paukov, M.; Kramberger, C.; Begichev, I.; Kharlamova, M.; Burdanova, M. Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics. Materials 2023, 16, 1276. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.V.; Kramberger, C. Phemenology of filling, investigation of growth kinetics and electronic properties for ap-plications of filled single-walled carbon nanotubes. Nanomaterials 2023, 13, 314. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Metal and Metal Halogenide-Filled Single-Walled Carbon Nanotubes: Kinetics, Electronic Properties, Engineering the Fermi Level. Nanomaterials 2023, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Burdanova, M.G.; Tsapenko, A.P.; Kharlamova, M.V.; Kauppinen, E.I.; Gorshunov, B.; Kono, J.; Lloyd-Hughes, J. A review of the terahertz conductivity and photoconductivity of nanotubes and related materials. Adv. Opt. Mater. 2021, 9, 2101042. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Nickelocene-filled purely metallic single-walled carbon nanotubes: Sorting and tuning the electronic prop-erties. Nanomaterials 2021, 11, 2500. [Google Scholar] [CrossRef]
- Sizikov, A.A.; Kharlamova, M.V.; Nikitin, P.I.; Nikitin, M.P. Non-viral locally injected magnetic vectors for in vivo gene delivery: A review of studies on magnetofection. Nanomaterials 2021, 11, 1078. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Eder, D. Tuning the Electronic Properties of Single-Walled Carbon Nanotubes by Filling with Electron Donor and Acceptor Compounds. Mater. Proc. 2021, 4, 67. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Eder, D. Carbon nanotubes: Synthesis, properties and new developments in research. In Synthesis and Applications of Nanocarbons; Arnault, J.C., Eder, D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 107–147. [Google Scholar]
- Kharlamova, M.V. Novel approaches to synthesis of double-walled carbon nanotubes. In Handbook of Carbon Nanotubes; Abraham, J., Kalarikkal, N., Thomas, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–21. [Google Scholar]
- Kharlamova, M.V.; Kramberger, C.; Rudatis, P.; Yanagi, K.; Eder, D. Characterization of the electronic properties of single-walled carbon nanotubes filled with an electron donor—Rubidium iodide: Multifrequency Raman and X-ray photoelectron spectros-copy studies. Phys. Status Solidi B 2019, 256, 1900209. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Rudatis, P.; Pichler, T.; Eder, D. Revealing the doping effect of encapsulated lead halogenides on single-walled carbon nanotubes. Appl. Phys. A 2019, 125, 320. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Novel approach to tailoring the electronic properties of single-walled carbon nanotubes by the encapsulation of high-melting gallium selenide using a single-step process. JETP Lett. 2013, 98, 272–277. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Yashina, L.V.; Eliseev, A.A.; Volykhov, A.A.; Neudachina, V.S.; Brzhezinskaya, M.M.; Zyubina, T.S.; Lukashin, A.V.; Tretyakov, Y.D. Single-walled carbon nanotubes filled with nickel halogenides: Atomic structure and doping effect. Phys. Status Solidi B 2012, 249, 2328–2332. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Sato, Y.; Suenaga, K.; Pichler, T.; Shiozawa, H. Chirality-dependent growth of single-wall carbon nanotubes as revealed inside nano-test tubes. Nanoscale 2017, 9, 7998–8006. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Brzhezinskay, M.M.; Vinogradov, A.S.; Suzdalev, I.P.; Maksimov, Y.V.; Imshennik, V.K.; Novichikhin, S.V.; Krestinin, A.V.; Yashina, L.V.; Lukashin, A.V.; et al. The formation and properties of one-dimensional FeHal2 (Hal = Cl, Br, I) nanocrystals in channels of single-walled carbon nanotubes. Nanotechnol. Russ. 2009, 4, 634–646. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Domanov, O.; Mittelberger, A.; Yanagi, K.; Pichler, T.; Eder, D. Endohedral Functionalization of Metallicity-Sorted Single-Walled Carbon Nanotubes. Proceedings 2020, 56, 33. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Domanov, O.; Mittelberger, A.; Yanagi, K.; Pichler, T.; Eder, D. Fermi level engineering of metallicity-sorted metallic single-walled carbon nanotubes by encapsulation of few-atom-thick crystals of silver chloride. J. Mater. Sci. 2018, 53, 13018–13029. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Mittelberger, A.; Yanagi, K.; Pichler, T.; Eder, D. Silver Chloride Encapsulation-Induced Modifications of Raman Modes of Metallicity-Sorted Semiconducting Single-Walled Carbon Nanotubes. J. Spectrosc. 2018, 2018, 5987428. [Google Scholar] [CrossRef]
- Kirchhoff, F.; Holender, J.M.; Gillan, M.J. Energetics and electronic structure of silver chloride. Phys. Rev. B 1994, 49, 17420–17423. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).