Abstract
I filled the single-walled carbon nanotubes (SWCNTs) with lead chloride, lead bromide, and lead iodide. The high-resolution transmission electron microscopy data proved the filling of SWCNTs. I investigated the electronic properties using Raman spectroscopy and X-ray photoelectron spectroscopy, and I showed the p-doping of SWCNT.
1. Introduction
Single-walled carbon nanotubes (SWCNTs) are filled with metal chalcogenides, metal halogenides, metals, and molecules. Metal halogenides are the largest group of compounds that are introduced inside SWCNTs. I filled many metal halogenides inside SWCNTs. Metal halogenides can be electron donors or electron acceptors. The filling methods of metal halogenides are the liquid phase method and the melt method. In this method, the compound is melted, and it is incorporated inside SWCNTs by capillary forces [1,2]. The SWCNTs filled with metal halogenides can find applications in nanoelectronics, sensors, catalysis, biomedicine, electrochemical energy storage, solar cells, and light emission. To apply the filled SWCNTs in these fields, the SWCNTs are investigated by spectroscopic techniques, such as Raman spectroscopy, near-edge X-ray absorption fine structure spectroscopy, X-ray photoelectron spectroscopy, and optical absorption spectroscopy. The investigations show that the filled SWCNTs have a doping effect on SWCNTs, and the doping effect depends on metal type, halogen type, filling ratio, and type of used carbon nanotubes [3,4,5,6]. In this work, I fill the SWCNTs with lead halogenides, and I investigate the electronic properties of filled SWCNTs by spectroscopic techniques. The investigations show that the encapsulated lead halogenides cause p-doping of SWCNTs. The doping effect depends on the halogen type. It is maximal for lead chloride, and it is minimal for lead bromide. This is caused by the different filling ratios of SWCNTs. Such filled SWCNTs can find applications in various fields, in particular in solar cells.
2. Experimental
The SWCNTs and lead halogenides are put into quartz ampoules, and sealed under vacuum. The heating of the system is performed to melt the salt and fill it inside SWCNTs. The following cooling causes the crystallization of the system. The synthesis is performed at temperatures that are 100 °C higher than the melting point of the compounds (Tmelting(PbCl2) = 501 °C, Tmelting(PbBr2) = 371 °C, and Tmelting(PbI2) = 402 °C).
3. Results
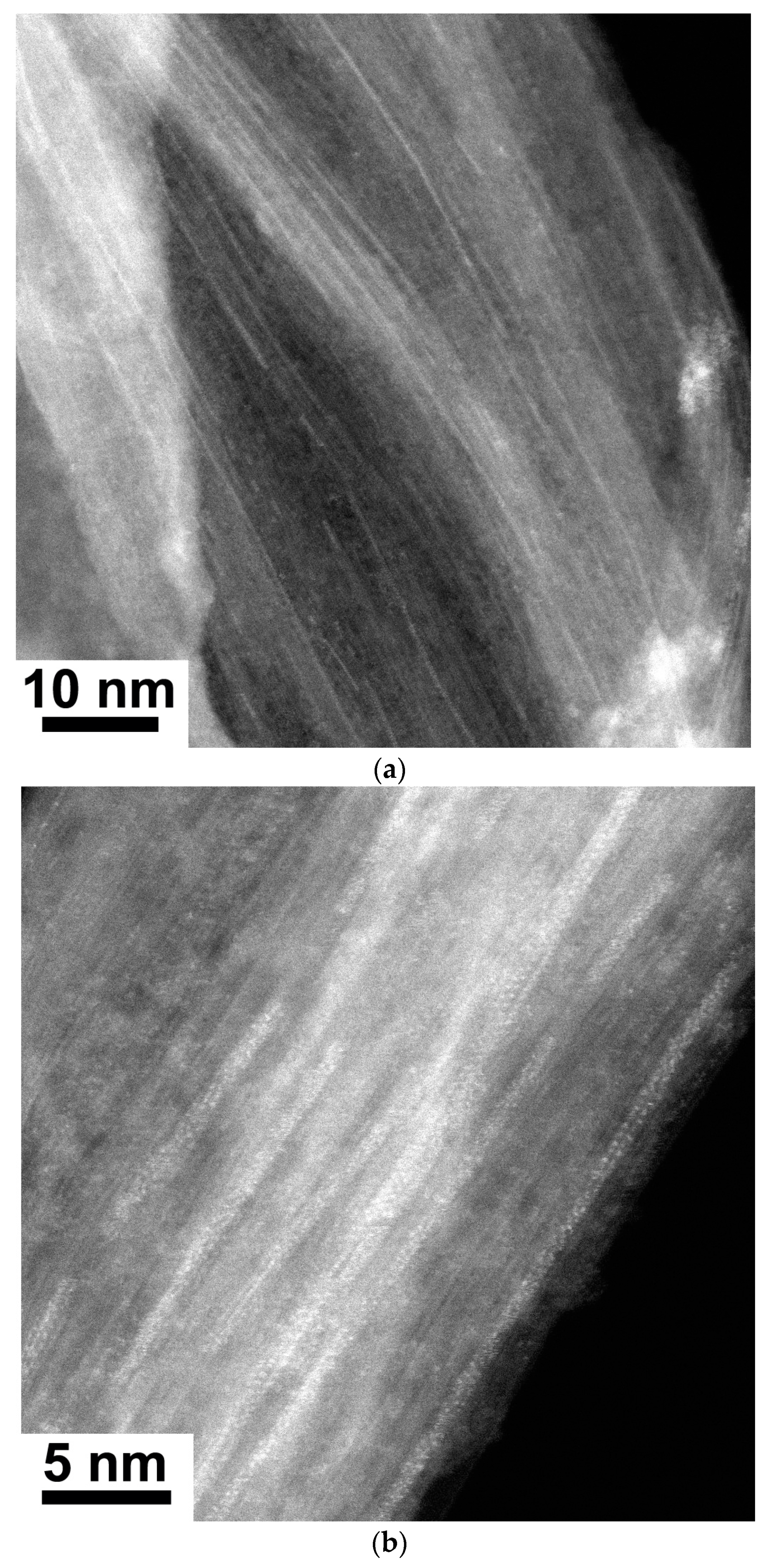
The data of high-resolution electron microscopy (HRTEM) of filled SWCNTs prove the filling of compounds inside SWCNTs. Figure 1 shows the HRTEM images of lead chloride-filled SWCNTs. In Figure 1a, one can see the bundle of lead chloride-filled SWCNTs. The white contrast lines are observed in the image, which corresponds to the filled channels of SWCNTs. Figure 1b shows individual SWCNT filled with lead chloride in the downright part of the image. The individual atoms of salts are visible in the image.
Figure 1.
The HRTEM image of bundle of lead chloride-filled SWCNTs (a), the image showing individual lead chloride-filled SWCNT (b).
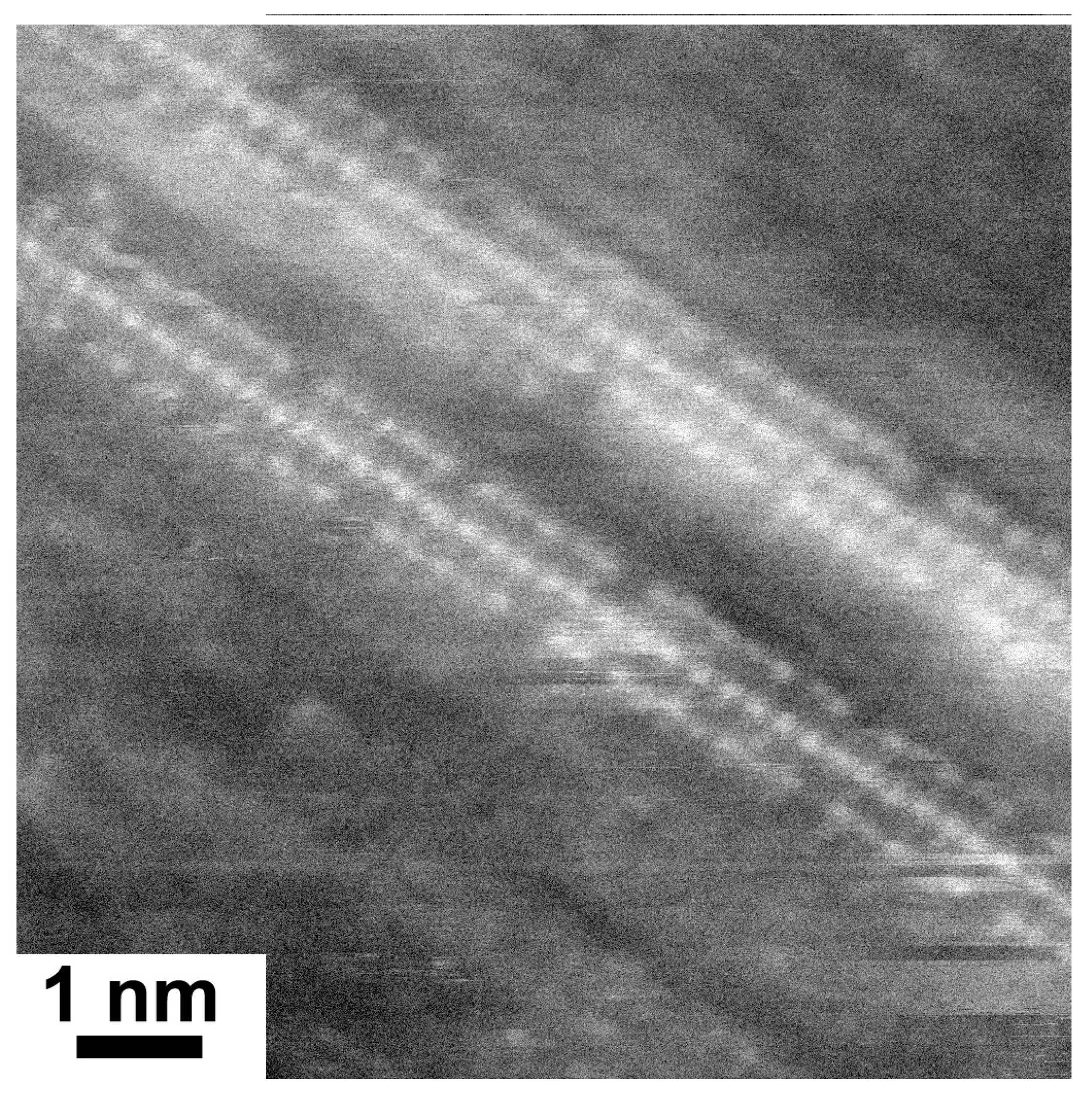
Figure 2 shows the HRTEM image of lead iodide-filled SWCNTs. Two filled SWCNTs are visible in the image. The atoms of salts are clearly recognized inside SWCNTs. They are visible as white dots. Three columns of atoms are observed within the walls of carbon nanotubes. The distances between three columns of atoms are equal.
Figure 2.
The HRTEM image showing two individual lead iodide-filled SWCNTs.
The investigations of lead halogenide-filled SWCNTs by spectroscopic techniques, such as Raman spectroscopy, and X-ray photoelectron spectroscopy showed that the introduced salts have a p-doping effect on the carbon nanotubes. The effect is different for different lead halogenides. Lead chloride has the maximal doping effect on SWCNTs, whereas lead bromide has the smallest doping effect on carbon nanotubes.
4. Conclusions
In this work, I filled the SWCNTs with lead halogenides. The HRTEM data proved the filling of compounds in the interior cavities of SWCNTs. The electronic properties were analyzed by Raman spectroscopy and X-ray photoelectron spectroscopy. The p-doping of carbon nanotubes was confirmed.
Funding
These studies were partly performed during the implementation of the project Building-up Centre for advanced materials application of the Slovak Academy of Sciences, ITMS project code 313021T081 supported by Research and Innovation Operational Programme funded by the ERDF.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I acknowledge Andreas Mittelberger (University of Vienna, Vienna, Austria) for the HRTEM measurements.
Conflicts of Interest
The author declares no conflict of interest.
References
- Monthioux, M. Filling single-wall carbon nanotubes. Carbon 2002, 40, 1809–1823. [Google Scholar] [CrossRef]
- Monthioux, M.; Flahaut, E.; Cleuziou, J.P. Hybrid carbon nanotubes: Strategy, progress, and perspectives. J. Mater. Res. 2006, 21, 2774–2793. [Google Scholar] [CrossRef]
- Vorfolomeeva, A.A.; Stolyarova, S.G.; Asanov, I.P.; Shlyakhova, E.V.; Plyusnin, P.E.; Maksimovskiy, E.A.; Gerasimov, E.Y.; Chuvilin, A.L.; Okotrub, A.V.; Bulusheva, L.G. Single-Walled Carbon Nanotubes with Red Phosphorus in Lithium-Ion Batteries: Effect of Surface and Encapsulated Phosphorus. Nanomaterials 2023, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Vorfolomeeva, A.A.; Pushkarevsky, N.A.; Koroteev, V.O.; Surovtsev, N.V.; Chuvilin, A.L.; Shlyakhova, E.V.; Plyusnin, P.E.; Makarova, A.A.; Okotrub, A.V.; Bulusheva, L.G. Doping of Carbon Nanotubes with Encapsulated Phosphorus Chains. Inorg. Chem. 2022, 61, 9605–9614. [Google Scholar] [CrossRef] [PubMed]
- Fedoseeva, Y.V.; Orekhov, A.S.; Chekhova, G.N.; Koroteev, V.O.; Kanygin, M.A.; Senkovskiy, B.V.; Chuvilin, A.; Pontiroli, D.; Riccò, M.; Bulusheva, L.G.; et al. Single-Walled Carbon Nanotube Reactor for Redox Transformation of Mercury Dichloride. ACS Nano. 2017, 11, 8643–8649. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.V.; Gurova, O.A.; Makarova, A.A.; Fedorenko, A.D.; Nikolenko, A.D.; Plyusnin, P.E.; Arenal, R.; Bulusheva, L.G.; Okotrub, A.V. Light-Induced Sulfur Transport inside Single-Walled Carbon Nanotubes. Nanomaterials 2020, 10, 818. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).