Abstract
Members of many species of the genus Gordonia are known for their ability to utilize compounds of different structures. The aim of the work was to study the ability of nine G. alkanivorans strains to degrade persistent organic pollutants and to analyze the genomic peculiarities of these strains. The genomes of nine Gordonia alkanivorans strains were sequenced and assembled. Based on the unique genes in the genomes, the strains can be divided into two subgroups. The strains can be used in biotechnologies of the environmental treatment as alkane degraders. Additionally, they all utilize benzoate.
1. Introduction
Gordonia strains are known for their ability to adapt to various environmental conditions and their extensive metabolic capabilities due to the remarkable plasticity of the genomes of this genus [1,2,3]. It can be said that Gordonia strains are ubiquitous.
Members of many species of the genus Gordonia are further known for their ability to utilize compounds of a different structure, including persistent organic pollutants. This makes Gordonia strains promising for application in the field of environmental biotechnology.
The species Gordonia alkanivorans was introduced in 1999 due to the isolation of a type strain of this species, HKI 0136T, from tar- and phenol-contaminated soil [4]. Currently, G. alkanivorans strains are known mainly as degraders of two types of compounds: thiophenes as a sulfur source [5,6] and alkanes as carbon and energy sources.
Previously, it was thought that thiophene catabolism in G. alkanivorans was only carried out using the operon dsz [5,7]. However, we have shown that there are G. alkanivorans strains that utilize thiophenes without dsz genes [8,9]. Alkane catabolism by G. alkanivorans strains is performed by P450 hydroxylases (CYP153); the absence of the alkB genetic system is characteristic of all the known representatives of this genus [8].
The aim of the work is to study the ability of nine G. alkanivorans strains to degrade persistent organic pollutants and to analyze the genomic peculiarities of these strains. The results will make it possible to assess the prospects for the application of these strains in biotechnologies for the remediation of contaminated soil ecosystems.
2. Materials and Methods
2.1. Bacterial Strains and Cultivation Conditions
We used nine bacterial strains (Table 1) isolated from oil-contaminated soils and the G. alkanivorans strain 135 [8,10] as a reference to investigate the catabolic properties.

Table 1.
Information about the strains used in the study.
The ability of the strains to utilize thiophenes as the only sulfur source was tested by the method described in [8]. The ability of the strains to grow on even alkanes (C6–C20) and aromatic compounds (naphthalene, phenol, benzoate, catechol) was tested using a mineral medium of the following composition: K2HPO4—8.71 g/L, 5 M NH4Cl solution—1 mL/L, 0.1 M Na2SO4 solution—1 mL/L, 62 mM MgCl2 solution—1 mL/L, 1 mM CaCl2 solution—1 mL/L, 0.005 mM of (NH4)6Mo7O24 × 4H2O solution, micronutrients—1 mL (micronutrient composition in g/L: ZnO—0.41 g, FeCl3 × 6H2O—5.4 g, MnCl2 × 4H2O—2 g, CuCl2 × 2H2O—0.17 g, CoCl2 × 6H2O—0.48 g, H3BO3—0.06 g), and pH 7.0. Alkanes were added at 7.5 mL/L, naphthalene, phenol, and benzoate at 1 g/L, and catechol at 0.1 g/L.
2.2. Genome Sequencing and Analysis
The genomic DNA of strains was isolated from a biomass grown on LB [11] agar using a DNeasy Blood and Tissue Kit (QIAGEN, 69506). Sequencing was performed on a MGI platform (DNBSEQ-G400) using the DNBSEQ-G400RS high-throughput sequencing set (FCL PE150) (2 × 150 bp). A paired-end library was prepared with the MGIEasy Universal DNA library prep set. The information on the generated data is presented in Table 2.

Table 2.
Number of sequencing data before and after filtration.
The raw reads were filtered using Trimmomatic v. 0.39 [12] and assembled using SPAdes v. 3.15.4 [13]. Contigs shorter than 500 bp were removed (Table 3).

Table 3.
Assembly metrics of the strains.
The average nucleotide identity (ANI) value with the type strain G. alkanivorans NBRC16433 (BACI00000000.1) was calculated using the EzBioCloud ANI Calculator (https://www.ezbiocloud.net/tools/ani, accessed on 14 April 2023) [14]. DNA–DNA hybridization (DDH) was calculated using the genome-to-genome distance calculator (GGDC) [15]. The genome was annotated with the NCBI prokaryotic genome annotation pipeline (PGAP) version 4.6 [16], Prokka [17] and RAST [18].
3. Results and Discussion
3.1. Identifying the Strains
Based on the results of the whole genome sequencing data, several strains were reidentified (Table 4).

Table 4.
Species identification of the strains.
Thus, all the strains reliably belong to Gordonia alkanivorans. The difference in the DDH value between the studied strains and the type of strain G. alkanivorans NBRC16433 indicates some heterogeneity of the species, but all the strains pass the species threshold by both ANI (>96%) and DDH (>70%).
3.2. Physiological and Biochemical Characteristics of Strains
On agarized rich media (LB), all the strains form small round colonies of a pink-orange color. When growing on mineral media with alkanes as a carbon source or thiophenes as a sulfur source, lighter orange colonies are formed. The color change possibly indicates that when growing on mineral media with energy sources that are difficult to access, microorganisms expend energy for basic metabolic processes and substrate utilization, but not for the biosynthesis of secondary metabolites (carotenoids) which give cells their color.
Crude oil is a complex mixture of components, these being aliphatic and aromatic hydrocarbons, as well as their sulfur-, nitrogen-, and oxygen-containing derivatives. In this regard, we assumed that the strains isolated from areas contaminated with crude oil may be capable of utilizing compounds of each of these groups. All strains were capable of utilizing alkanes from C10 to C20 and benzoate; some strains are capable of using dibenzothiophene (DBT) as the sole source of sulfur (Table 5).

Table 5.
Substrate specificity profile of strains.
Thus, there is still no strain of G. alkanivorans capable of utilizing PAHs (naphthalene) or their metabolites (catechol) known at this time.
3.3. Peculiarities of G. alkanivorans Genome Organization and Pangenome Analysis
Of the nine strains of G. alkanivorans, two (strains 142 and 152) have plasmid elements. The plasmid of strain 142 (p142) is 67,219 bp in length; the plasmid of strain 152 (p152) is 44,937 bp. We assume that they are circular because Gordonia in general is not characterized by the maintenance of large linear plasmids as, for example, in rhodococci.
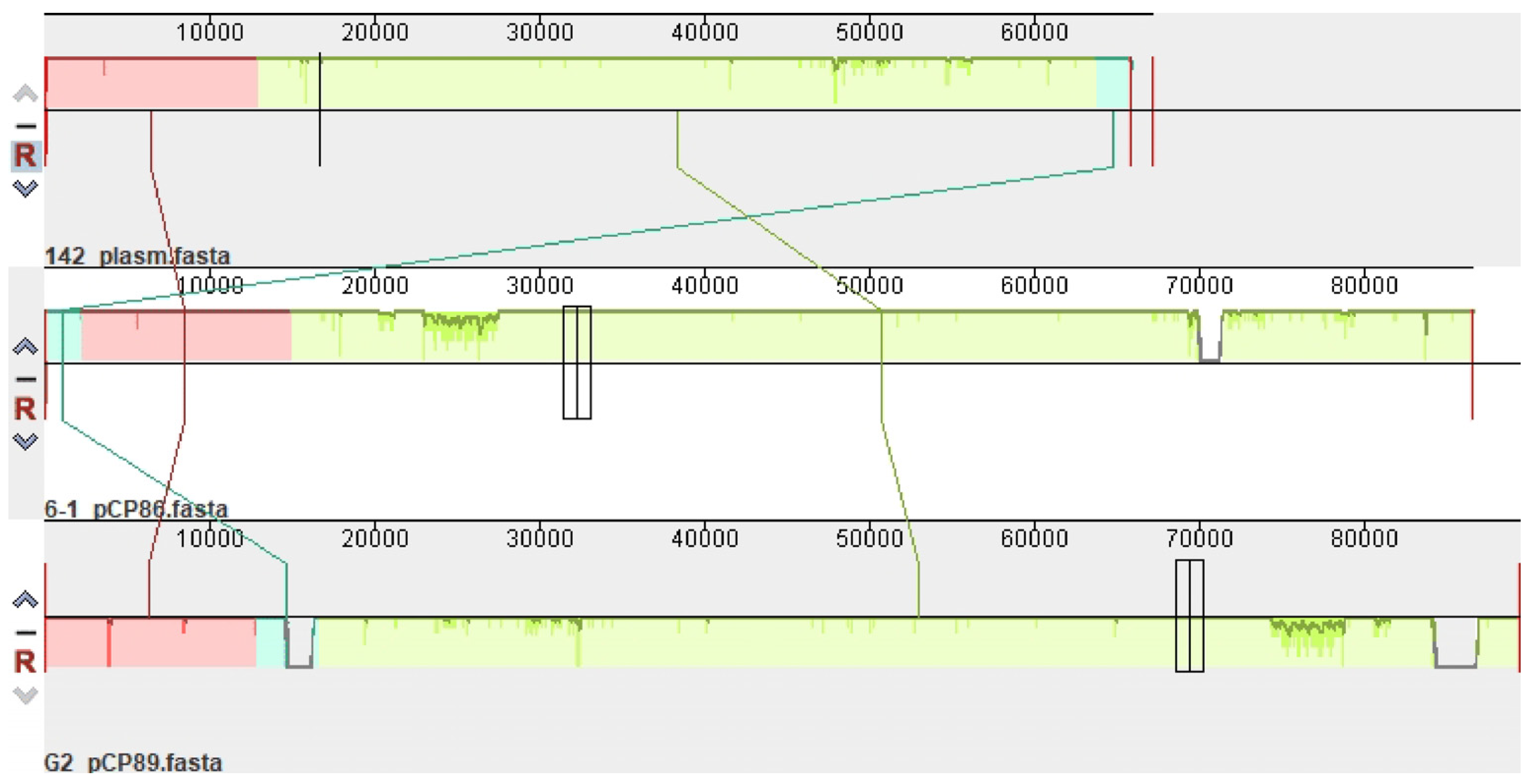
In total, 98% of the entire length of plasmid p142 is a 99% repeat of plasmids pCP89 (CP094666.1) of the Gordonia amicalis G2 strain (percent identity (PI) 99.70%) and pCP86 (CP096597.1) of the G. amicalis 6-1 strain (PI 99.98%) (Figure 1). It is interesting to note this relatedness between plasmids whose hosts are strains of different species.
Figure 1.
Mauve alignment demonstrating relatedness of the plasmids p142 (strain G. alkanivorans 142), pCP86 (G. amicalis 6-1) and pCP89 (G. amicalis G2). Vertical bars mark boundaries between elements.
The plasmid of strain 152 has a relatedness with the plasmid pG135. The ANI value between the plasmids p142 and p152 is 80.00%.
Based on the results of genome analysis and individual unique genes, the strains can be divided into two groups within the species. The representatives of the first group (strains 129, 144, 132, 133) are characterized by unique genes of tyrocidine and gramicidin biosynthesis. The representatives of the second group have a greater catabolic potential: the genomes of the strains contained (1) operons for the biosynthesis of steroid compounds, (2) additional copies of genes involved in dibenzothiophene catabolism, and (3) genes of the aromatic compound catabolic process: cytochrome P450-pinF2 and phenol hydroxylase P5. All the studied G. alkanivorans strains lack alkB genes in their genomes; therefore, we assume that the ability to utilize alkanes in these strains is controlled by the CYP153 genetic system. The CYP153 hydroxylases have at least a 99% identity between strains and contain one amino acid substitution each: one hydroxylase has A/T variants at position 239, the other hydroxylase has A/S variants at position 7, and they are in the first and second strain groups, respectively.
4. Discussion
In general, we can say that the strains of the G. alkanivorans species are similar in terms of their physiological properties and degradative potential. They utilize alkanes with different chain lengths, and, among the compounds with an aromatic structure, benzoate is available to them. Nevertheless, representatives of this species have no metabolic pathways of naphthalene degradation.
It is interesting to note that Gordonia plasmids do not appear to be species-specific. The function of plasmid p142 in strain 142 is currently unclear, but the fact that copies of the plasmid have been observed in members of another species (G. amicalis) may indicate the importance of this plasmid for the vital activity of Gordonia. We plan to obtain a plasmid-free eliminant of strain 142 in the future in order to obtain a better understanding of the functions of this plasmid. At this point, we can assume that plasmids such as p142 are required by strains for metal transport and resistance.
Based on the pangenome analysis, we were able to trace some regularities of strain distribution within the species. Nine representatives of G. alkanivorans can be divided into two subgroups, which are distinguished by unique genes. Considering the presence of a greater number of catabolic genes and operons in the representatives of the second subgroup (strains 96, 134, 142, 12, 152), we can assume that these strains can be promising in the field of biotechnologies for the purification of the environment from, for example, steroid compounds.
5. Conclusions
The genomes of nine strains of Gordonia alkanivorans isolated from oil-contaminated soils were sequenced and assembled. The genomes are about 5 Mb in size. Some of the strains contain plasmids, but the functions of these plasmids are currently not fully understood. A pangenome analysis of the strains has shown that, within the species, there are differences between the strains, allowing them to be conditionally divided into two subgroups according to the unique genes of each strain. The strains can be used in environmental treatment biotechnologies as alkane degraders. Additionally, all of the strains also utilize benzoate.
Author Contributions
Conceptualization, Y.D. and A.V.; methodology, E.F., A.B. and I.S.; software, Y.D.; validation, A.B., Y.D. and I.S.; formal analysis, A.V.; investigation, E.F., L.S. and A.V.; data curation, Y.D.; writing—original draft preparation, E.F. and Y.D.; writing—review and editing, A.B. and A.V.; visualization, E.F.; supervision, Y.D.; project administration, Y.D.; funding acquisition, Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Russian Science Foundation, grant number 22-74-10082.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The whole-genome sequences of the strains can be found under BioProject number PRJNA955828, BioSamples SAMN34194808-SAMN34194816.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arenskötter, M.; Bröker, D.; Steinbüchel, A. Biology of the Metabolically Diverse Genus Gordonia. Appl. Environ. Microbiol. 2004, 70, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.-T.; Lu, H.-L.; Lin, J.-L.; Huang, W.-S.; Arun, A.B.; Young, C.-C. Phylogenetic analysis of members of the metabolically diverse genus Gordonia based on proteins encoding the gyrB gene. Res. Microbiol. 2006, 157, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.-T.; Young, C.-C. Rapid detection and identification of the metabolically diverse genus Gordonia by 16S rRNA-gene-targeted genus-specific primers. FEMS Microbiol. Lett. 2005, 250, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kummer, C.; Schumann, P.; Stackebrandt, E. Gordonia alkanivorans sp. nov., isolated from tar-contaminated soil. Int. J. Syst. Evol. Microbiol. 1999, 49, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Marques, S.; Matos, J.; Tenreiro, R.; Gírio, F.M. Dibenzothiophene desulfurization by Gordonia alkanivorans strain 1B using recycled paper sludge hydrolyzate. Chemosphere 2008, 70, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Aminsefat, A.; Rasekh, B.; Ardakani, M.R. Biodesulfurization of dibenzothiophene by Gordonia sp. AHV-01 and optimization by using of response surface design procedure. Microbiology 2012, 81, 154–159. [Google Scholar] [CrossRef]
- Jaishankar, J.; Singh, P.; Srivastava, P. Draft Genome Sequence of a Biodesulfurizing Bacterium, Gordonia sp. Strain IITR100. Genome Announc. 2017, 5, e00230-17. [Google Scholar] [CrossRef] [PubMed]
- Delegan, Y.; Kocharovskaya, Y.; Frantsuzova, E.; Streletskii, R.; Vetrova, A. Characterization and genomic analysis of Gordonia alkanivorans 135, a promising dibenzothiophene-degrading strain. Biotechnol. Rep. 2021, 29, e00591. [Google Scholar] [CrossRef] [PubMed]
- Frantsuzova, E.; Delegan, Y.; Bogun, A.; Sokolova, D.; Nazina, T. Comparative Genomic Analysis of the Hydrocarbon-Oxidizing Dibenzothiophene-Desulfurizing Gordonia Strains. Microorganisms 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Delegan, Y.; Valentovich, L.; Vetrova, A.; Frantsuzova, E.; Kocharovskaya, Y. Complete Genome Sequence of Gordonia sp. 135, a Promising Dibenzothiophene- and Hydrocarbon-Degrading Strain. Microbiol. Resour. Announc. 2020, 9, e01450-19. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on Lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).