Abstract
Most wine fermentations are completed in successive batches during a 2 month harvest period. Red wine fermentations are usually completed in 10 to 14 days, while white wine fermentations are completed in 21 to 24 days. The demand for resources—equipment, water, energy, labor, etc.—in a short time motivates the precise, automated control of the fermentation process. While the effects of temperature on wine chemistry are well studied, few advanced control strategies have been developed for the precise control of temperature for wine chemistry research. In this study, a nonlinear model predictive controller (NMPC) was developed to determine the temperature to achieve the desired fermentation rate. The controller was combined with a pulse cooling and heating strategy to improve the energy efficiency of temperature control. The feasibility of this approach is demonstrated by simulations and experiments in 15 L fermentations.
1. Introduction
In the production of wine, the entire primary fermentation processing step is performed within 1 to 3 months after grapes are harvested, depending on the type of wine produced. The demand for resources—equipment, water, energy, labor, etc.—in a short time period motivates the precise control of the fermentation process. While much research has been performed to evaluate the effect of the fermentation temperature on wine chemistry [1,2,3,4,5,6], in this paper, the effect of specific fermentation kinetics on the resulting wine characteristics and the reproduction of the results at commercial volumes with a robust control strategy is investigated. To our knowledge, there is only one example of wine fermentation with a controlled fermentation rate, performed by Saller [7] in 1958. In [7], the fermentation rate of a 1500 L white juice fermentation was controlled to 1 °Brix per day (which is 4° Öschle per day, as reported [7]). An external heat exchanger was used to quickly change the temperature of the juice. Around the end of the lag phase, a large input of energy was needed to cool the fermentation down to 2 °C. After around 10 days, the temperature was increased up to 14 °C. A subsequent evaluation by a wine kinetic model [8] indicated the Saller fermentation had a very high viability and a low specific maintenance rate. The duration of fermentation was 30 days. In the commercial production of wine today, a fermentation time of 30 days is considered atypically long and will limit the ability to reuse fermentors as more grapes are harvested. Additionally, the use of temperatures down to 2 °C requires very cold refrigerant, making the energy usage and associated costs high. In this work, a fermentation rate of 2.5 °Brix per day was chosen to increase the minimum temperature and allow the fermentation to complete in approximately 10 days.
In general, the control and optimization of wine fermentation is a challenge because of the nonlinear process control needed, incomplete descriptions by first principle models and the ability to measure important parameters’ reliability with online sensors. Nonlinear model predictive control (NMPC) is a control strategy that uses a mathematical model to predict the future response of the system to different control actions and find the control action that minimizes an object function. NMPC has been applied to multiple different fermentation processes including fed-batch penicillin production [9], fed-bactch production of Escherichia coli [10], fed-batch Chinese hamster ovary mammalian cell production [11], cultivation of hybridoma cells for production of monoclonal antibodies [12], fed-batch Saccharomyces cerevisiae for the production of ethanol [13] and continuous ethanol production fermentation using Zymomonas mobilis [14]. In this work, NMPC is developed for the fermentation of wine and demonstrated in a 15 L fermentor.
2. Materials and Methods
2.1. Fermentation Setup
A diagram of the fermentation setup is shown in Figure 1.
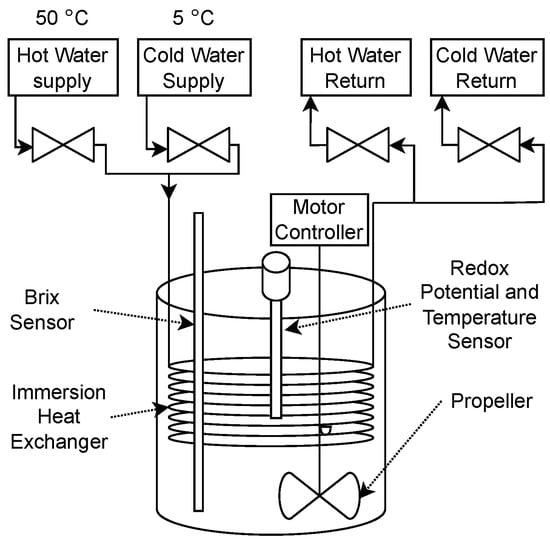
Figure 1.
Fermentation setup diagram.
An immersion heat exchanger (40474, Northern Brewer, Roseville, MN, USA) was immersed in a 25 L bucket and connected to four valves (BZW-15-24VAC, Electric Solenoid Valves, Islandia, NY, USA). Either hot or cold water is supplied to the heat exchanger and returned to the water loop. The hot and cold water supply was measured throughout the experiment and on average was about 50 °C and 5 °C, respectively. The density, measured as °Brix, was measured with a differential pressure method, as described in previous work [15]. A platinum electrode 120 mm Arc oxidation-reduction potential (ORP) probe (Hamilton Company, Reno, NV, USA) was inserted in the center of the bucket. The ORP probe contained a thermistor for the measurement of temperature. Continuous mixing was provided with a speed controlled propeller (ADI 1012, Applikon Biotechnology, Foster City, CA, USA) set to 120 revolutions per minute to provide adequate mixing in the tank. The propeller had to be offset from the center to accomodate the sensors and heat exchanger.
2.2. Nonlinear Model Predictive Control
Figure 2 shows the nonlinear model predictive control strategy. All measurements were stored in a database (PI, OSIsoft, San Leandro, CA, USA). The timestep used was 30 min with a prediction horizon of 30 min. Every 30 min, the new and previous measurements of density and temperature were used to estimate the parameters of a wine kinetic model. The updated parameters were inputed into an optimization block.
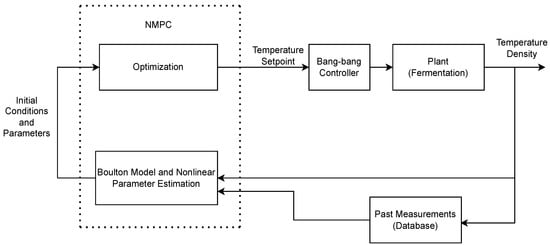
Figure 2.
NMPC block diagram.
In the optimization block, the temperature that minimizes the mean squared error (MSE) between the predicted and desired density is found and used as the setpoint throughout the 30 min time horizon.
2.3. Wine Kinetic Model and Parameter Estimation
The Boulton model [8] describes the kinetics of juice to wine based on commonly used wine yeast strains of Saccharomyces Cerevisiae. In this work, the Boulton model used was modified for an assimilable nitrogen term in the yeast growth rate and to accept measurements of temperature. The modeling and parameter estimation is further described in [16].
2.4. Pulse Temperature Control
A bang-bang controller was used to control the fermentation temperature to the setpoint determined by NMPC. Every 120 s, the controller compared the current temperature of the juice to the setpoint and opened the appropriate hot or cold valves for 20 s. This bang-bang controller demonstrates a simple pulse cooling and heating strategy, since an off time of 100 s is guaranteed to allow the heat to transfer between the hot or cold water and the juice through the immersion heat exchanger. The energy savings of pulse cooling and heating to the control of temperature in jacketed fermentors in commercial winemaking is significant (Robert Coleman, private communications).
2.5. Wine Fermentations
White grape concentrate was diluted to 15 L of 24 °Brix juice. An amount of 100 mg/L of juice mineral nutrients (Thiazote pH, Laffort, Petaluma, CA, USA) was added to ensure no vitamin deficiencies were present. Yeast rehydration nutrients (250 g/mL) (GO-Ferm, Scott Laboratories Inc., Petaluma, CA, USA) were added to 250 g/mL of yeast (EC-1118, Lalvin, France). After the yeast was rehydrated according to the manufacturer’s recommendation, the yeast mixture was added to the fermentation. Three control fermentations with constant temperatures of 21 °C, 23 °C and 25 °C were performed. The temperature control was started 60 h after inoculation. After the three control fermentations, one fermentation was performed with the NMPC strategy. The desired fermentation rate was set to 2.5 Brix per day with a lag of 20 h.
3. Results
Figure 3A,B shows the measured °Brix, temperature and ORP for a constant temperature fermentation, while Figure 3C,D shows the °Brix, temperature and ORP for the MPC fermentation. Due to an equipment failure, measurements from the NMPC fermentation were only collected from 24 to 9 °Brix.
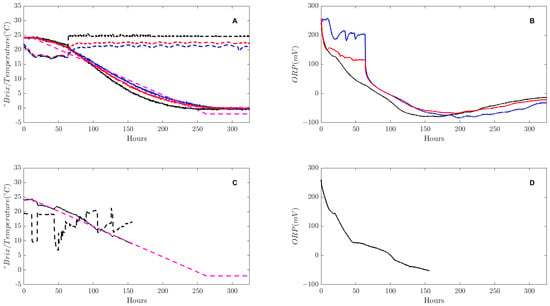
Figure 3.
The measured °Brix and temperature (A) and ORP (B) at 21 °C (blue) 23 °C (red) and 25 °C (black). The measured °Brix, temperature and ORP for the MPC fermentation are shown in (C,D). The dashed purple line in A and C indicate the desired linear °Brix.
From the °Brix measurements in Figure 3A, fermentations with constant temperatures show the sigmoidal shape characteristic of wine fermentations, while Figure 3C shows a linear fermentation rate from 24 to 9 °Brix, demonstrating clearly the extent the NKB (Nelson–Knoesen–Boulton) NMPC can control the fermentation kinetics.
4. Discussion
An important feature of any process control strategy in winemaking is the ability to adjust to differences between fermentations. Due to the large variation in initial chemical compositions of grapes and juices, the behavior of fermentations is unpredictable and variable. The NKB NPMC strategy demonstrated in this work is based on real-time measurements of density and temperature and is applicable to both research and commercial scale fermentations.
The ability to control fermentation rates will be an integral part in the management of stuck and sluggish fermentations and energy for refrigeration. Real-time measurements and modeling can be used to diagnosis fermentations that are not predicted to finish, switching temperature control to an NPMC to periodically select a temperature that ensures a complete fermentation. Additionally, since the fermentation rate is directly proportional to the heat release, control of the fermentation rate enables the optimal shifting of refrigeration loads across concurrent fermentations in a winery. Extending the NKB NPMC to an economic NPMC (ENMPC), the controller would also consider the current electricity rates, enabling economic optimization in the management of fermentation temperature within the constraints determined by a winemaker. Combined with pulse cooling and heating, ENMPC has the potential to lead to significant economic savings in wine fermentations.
As a secondary result of this study, the ORP was measured in all fermentations. ORP has been used in previous work to characterize vineyard site differences in Pinot noir fermentations [17] and as an indicator of microbial activity and the prevention of H2S [15]. The control of ORP has also been shown to accelerate the tailing section of some fermentations [18], making ORP an important variable to consider in the control of the fermentation rate. Additionally, the rate of decline and magnitude of the minimum point of ORP has been shown to be a function of temperature in isothermal fermentations [19]. Future development and application of mathematical models describing fermentation kinetics may be important to include ORP as a variable that captures both yeast activity and differences in juice composition.
5. Conclusions
A nonlinear model predictive control (NMPC) strategy to select the temperature setpoint that linearizes the rate of wine fermentation to 2.5 °Brix per day was developed and validated in a 15 L fermentation from 24 to 9 °Brix. The NMPC strategy used measurements of density and temperature to find the temperature setpoint that minimized the mean squared error between the predicted and desired density. Future work can demonstrate this at commercial volumes and incorporate economic models to enable the management of winery refrigeration loads.
Author Contributions
Conceptualization, J.N.; methodology, J.N. and R.B.; formal analysis, J.N.; investigation, J.N.; data curation, J.N.; writing—original draft preparation, J.N.; writing—review and editing, J.N., A.K. and R.B.; visualization, J.N.; supervision, A.K. and R.B.; funding acquisition, A.K. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
The Rodgers University fellowship in Electrical and Computer Engineering (J.N.) and the Stephen Sinclair Scott Endowment (R.B.). Partial funding provided by Opus One Winery.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NMPC | Nonlinear Model Predictive Controller |
| ENMPC | Economic Model Predictive Controller |
| MSE | Mean Squared Error |
| ORP | Oxidation-Reduction Potential |
| NKB | Nelson-Knoesen-Boulton |
References
- Boulton, R.; Singleton, V.; Bisson, L.; Kunkee, R. Principles and Practices of Winemaking; Springer: New York, NY, USA, 2013. [Google Scholar]
- Swiegers, J.; Bartowsky, E.; Henschke, P.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Cliff, M.; Girard, B.; Kopp, T.G. Influence of Fermentation Temperature on Composition and Sensory Properties of Semillon and Shiraz Wines. Am. J. Enol. Vitic. 2001, 52, 235–240. [Google Scholar] [CrossRef]
- Loureiro, V. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Saller, W. Control of Cold Fermentation. Am. J. Enol. Vitic. 1958, 9, 41–48. [Google Scholar] [CrossRef]
- Boulton, R. The Prediction of Fermentation Behavior by a Kinetic Model. Am. J. Enol. Vitic. 1980, 31, 40–45. [Google Scholar] [CrossRef]
- Ashoori, A.; Moshiri, B.; Khaki-Sedigh, A.; Bakhtiari, M.R. Optimal control of a nonlinear fed-batch fermentation process using model predictive approach. J. Process Control 2009, 19, 1162–1173. [Google Scholar] [CrossRef]
- Santos, L.; Dewasme, L.; Coutinho, D.; Wouwer, A.V. Nonlinear model predictive control of fed-batch cultures of micro-organisms exhibiting overflow metabolism: Assessment and robustness. Comput. Chem. Eng. 2012, 39, 143–151. [Google Scholar] [CrossRef]
- Craven, S.; Whelan, J.; Glennon, B. Glucose concentration control of a fed-batch mammalian cell bioprocess using a nonlinear model predictive controller. J. Process Control 2014, 24, 344–357. [Google Scholar] [CrossRef]
- Dewasme, L.; Fernandes, S.; Amribt, Z.; Santos, L.; Bogaerts, P.; Vande Wouwer, A. State estimation and predictive control of fed-batch cultures of hybridoma cells. J. Process Control 2015, 30, 50–57. [Google Scholar] [CrossRef]
- Chang, L.; Liu, X.; Henson, M.A. Nonlinear model predictive control of fed-batch fermentations using dynamic flux balance models. J. Process Control 2016, 42, 137–149. [Google Scholar] [CrossRef]
- Ajbar, A.; Ali, E. Study of advanced control of ethanol production through continuous fermentation. J. King Saud Univ. -Eng. Sci. 2017, 29, 1–11. [Google Scholar] [CrossRef]
- Nelson, J.; Coleman, R.; Chacón-Rodríguez, L.; Runnebaum, R.; Boulton, R.; Knoesen, A. Advanced Monitoring and Control of Redox Potential in Wine Fermentation across Scales. Fermentation 2022, 9, 7. [Google Scholar] [CrossRef]
- Nelson, J.; Boulton, R.; Knoesen, A. Automated Density Measurement with Real-Time Predictive Modeling of Wine Fermentations. IEEE Trans. Instrum. Meas. 2022, 71, 1–7. [Google Scholar] [CrossRef]
- Walker, G.A.; Nelson, J.; Halligan, T.; Lima, M.M.M.; Knoesen, A.; Runnebaum, R.C. Monitoring Site-Specific Fermentation Outcomes via Oxidation Reduction Potential and UV-Vis Spectroscopy to Characterize “Hidden” Parameters of Pinot Noir Wine Fermentations. Molecules 2021, 26, 4748. [Google Scholar] [CrossRef] [PubMed]
- Killeen, D.J.; Boulton, R.; Knoesen, A. Advanced Monitoring and Control of Redox Potential in Wine Fermentation. Am. J. Enol. Vitic. 2018, 69, 394–399. [Google Scholar] [CrossRef]
- Kukec, A.; Berovi, M. The Role of On-line Redox Potential Measurement in Sauvignon blanc Fermentation. Food Technol. Biotechnol. 2002, 40, 49–56. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).