Abstract
This study investigated the characteristics of banana peel biochar (BPB) produced from two particle sizes of banana waste at varying temperatures. The biochar yield decreased with increasing temperature and decreasing particle size. Physicochemical analysis showed the presence of amorphous carbon, fullerene, and chaolite in BPB produced at 500 °C. X-ray fluorescence(XRF) analysis showed high carbon, potassium, and chlorine percentages in BPB. BPB was found to be a promising adsorbent for copper (Cu2+) removal, with a maximum adsorption capacity of 54.9 mg/g and optimal Cu2+ concentrations for adsorption at 450 and 550 mg/L.
1. Introduction
Municipal solid waste generation in Lebanon has increased with the improvements in living standards, population growth, and urbanization, resulting in a yearly production of 2.55 million tons of waste. The majority of waste is organic matter, and traditional disposal methods include landfilling, incineration, and composting [1,2]. The cost of solid waste management in Lebanon is approximately USD 420 million per year and the improper handling of waste results in environmental damages of USD 66.5 million annually [3]. Dumped organic waste harms human health and the environment, producing greenhouse gases, leachate, and disease vectors [4]. Banana peels, which make up a significant portion of waste, can be transformed into high-value-added products like biochar [5,6]. Biochar has a wide surface area and an abundance of functional groups, making it useful for the adsorption of organic pollutants [7,8]. Pyrolysis, the carbonization process used to produce biochar, greatly affects its physicochemical properties, such as porosity, surface area, and adsorption capacity [9,10]. Therefore, selecting the appropriate pyrolysis temperature is crucial for biochar production [11].
The main goal of this study is to produce biochar from banana peels under various experimental conditions and to test their efficiency in the adsorption of copper from synthetic solutions. The specific objectives of this experimental investigation are (i) the assessment of the impact of the banana peels particle size and pyrolysis temperatures on the characteristics of the produced biochar, and (ii) the determination of the efficiency of this biochar in eliminating copper from synthetic solution under various experimental conditions.
2. Materials and Methods
2.1. Biochar Preparation
Banana peels were used in our experimental procedure. The peels were collected from a Lebanese kitchen waste, washed, air-dried, washed again, and dried in an oven. They were then grinded and sieved into 1 mm and 3 mm particles. The two types of banana peel waste were pyrolyzed at different temperatures (200–600 °C) and the resulting biochar was weighed to calculate the biochar yield. The experiments were carried out at a constant heating rate (5 °C/min) and residence time (1 h). The biochar yield was calculated using a formula (Equation (1)). Each experiment was performed three times and the mean values were reported. The standard deviation for all assays was less than 3%.
where mb and ma refer to the weight (g) of the sample before and after the pyrolysis process, respectively.
2.2. Biochar Characterization
The physicochemical properties of the produced biochar were obtained using different techniques, such as ash content, humidity ratio, pH, electrical conductivity, XRD analysis, SEM and XRF. These techniques were used to determine the crystal structure, surface area, morphology, chemical composition, and functional groups present in the biochar.
2.3. Adsorption Experiment
To prepare copper solutions, CuSO4.5H2O (BDH laboratory supplies, Poole, UK) was dissolved in ultra-pure water to obtain a mother solution of Cu2+ (1000 mg L−1), which was stored at 4 °C with HCl. The concentration of Cu2+ was determined using flame atomic absorption spectrometry (Beijing Rayleigh Analytical Instrument Corporation (BRAIC),FAAS, Rayleigh, WFX-200 AA Spectrophotometer Beijing, China,). Adsorption experiments were conducted using the best-synthesized biochar. Briefly, 0.5 g of biochar was shaken in 50 mL of Cu2+ solution (50–550 mg L−1) for one hour at 220 rpm. After filtration, the residual metal concentration was determined using flame atomic absorption spectrometry. Experiments were performed in triplicate and a blank solution was used for quality control.
3. Results
3.1. Biochar Yield
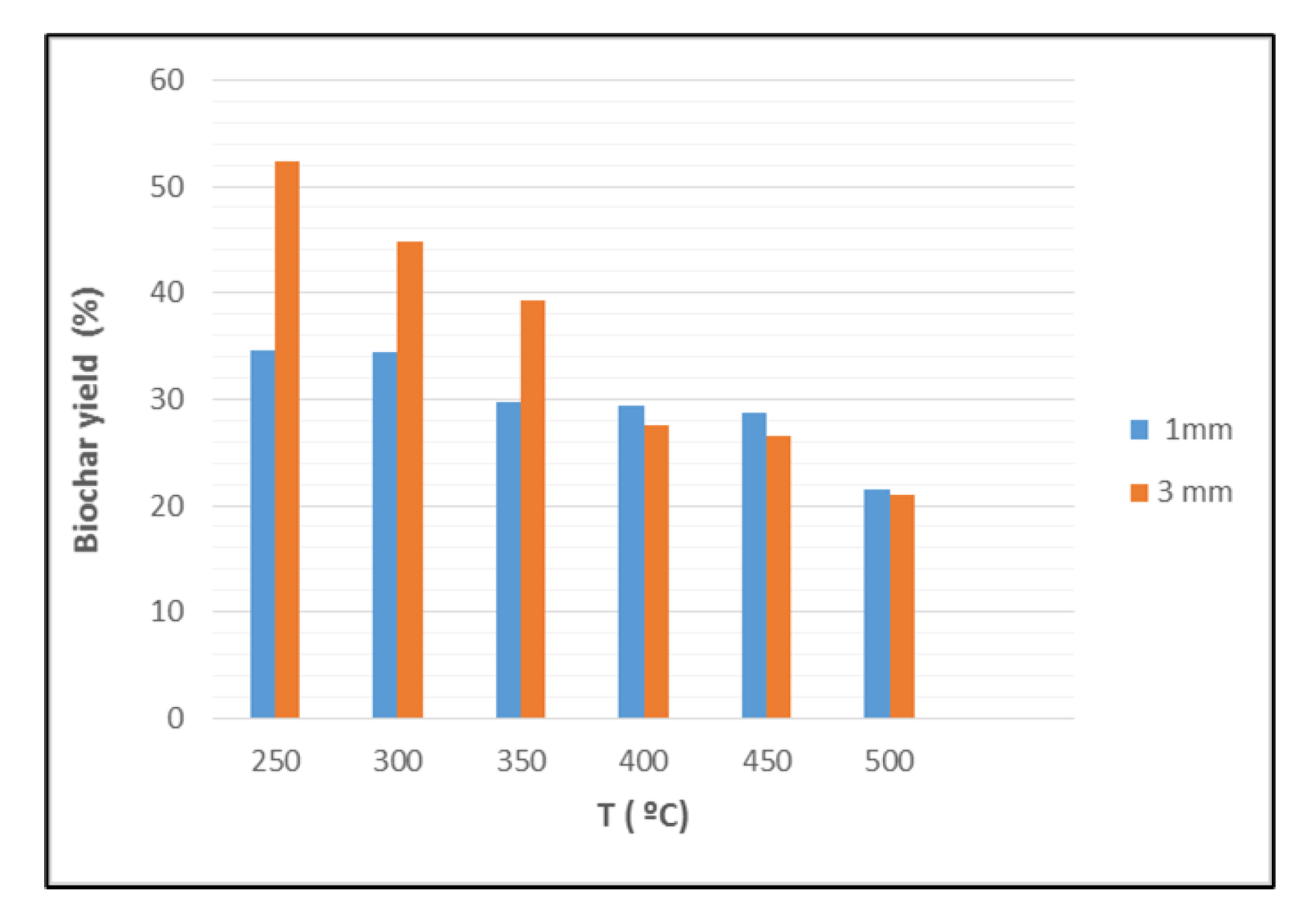
The biochar yield decreases from 52% to 25% when produced at 250 °C from two different grain sizes of BP (Figure 1). An increase in the pyrolysis temperature leads to a reduction in biochar yield, likely due to the conversion of biomass composed from cellulose, hemicellulose, and lignin into gases and bio-oil at high temperatures. Particle size, temperature, and heating rate all interact to affect the biochar yield. Lower temperatures lead to higher biochar yields because of faster primary decomposition and devolatilization. The 1 mm grain size produced ash at a lower temperature (200 °C) than the 3 mm grain size (350 °C) due to differences in surface reactivity and other grain properties.
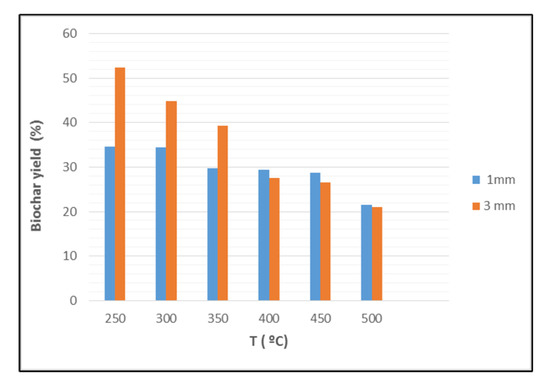
Figure 1.
Effect of banana peels particles size and pyrolysis temperature on the biochar yield.
3.2. Ash Content
The ash content of BPB was 15.76% and 17.29% for particle sizes of 1 mm and 3 mm, respectively, at 600 °C.
3.3. pH and Electrical Conductivity
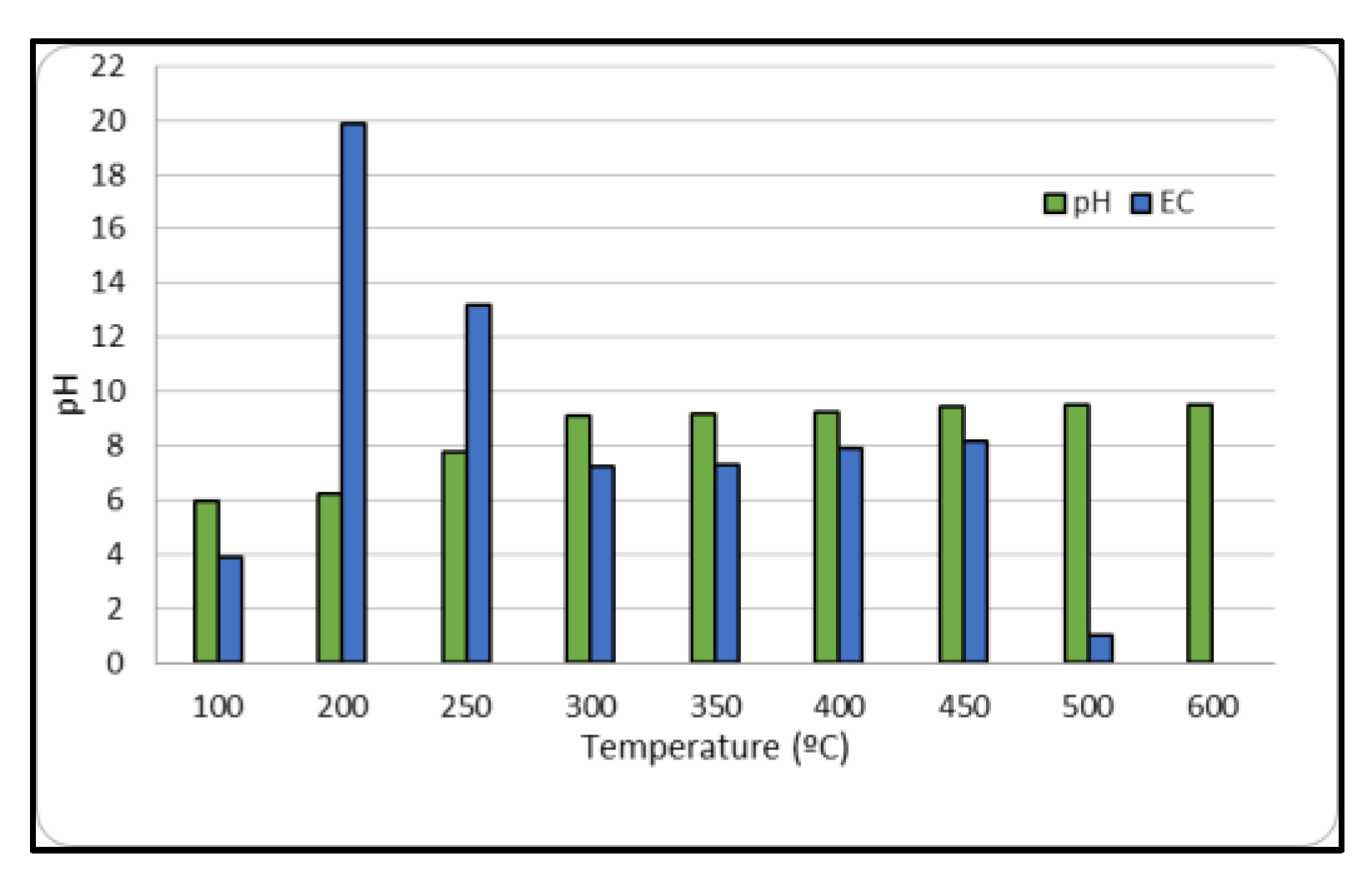
Figure 2 presents the obtained values of pH and electrical conductivity of BPB for the different temperatures. It shows that pH of biochar increases with carbonization temperature due to the formation of carbonates and inorganic alkalis. Biochar pH values range from 6.5 to 11, with BPB at T = 400 °C having a remarkably high pH value of 11. Electrical conductivity values also increase with pyrolysis temperature, but the values obtained in this study are lower than those reported in other studies [12]. This difference could be explained by the initial organic waste used and the adopted experimental conditions.
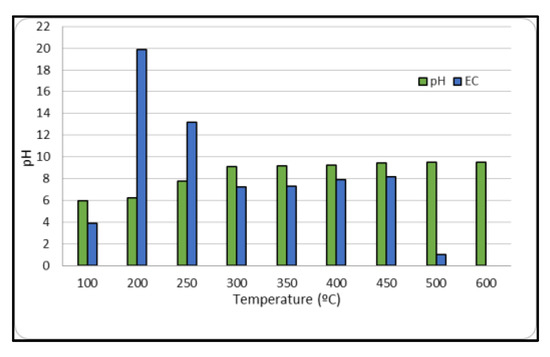
Figure 2.
pH and EC of BPB at different temperatures.
3.4. X-ray Diffraction (XRD)
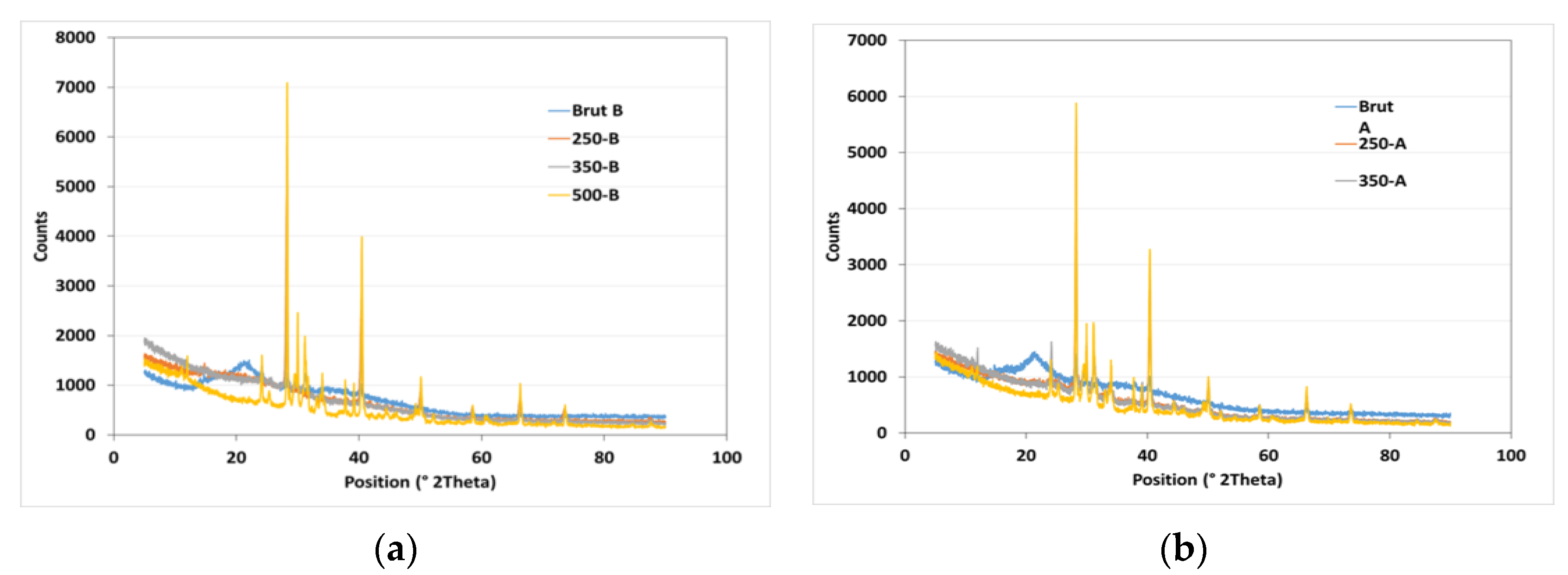
X-ray diffraction (XRD) analysis was used to study the structural order in carbon solids derived from banana peels biochar (BPB) at different pyrolysis temperatures and particle sizes. The loading of graphitic basal planes is observed at a 2θ value of 25°. The XRD pattern shows a different profile at 500 °C due to the appearance of chaolite and fullerene. Banana peel is thermally decomposed into chaolite and fullerene (Figure 3). The XRD peak intensities increase with increasing temperature, indicating the successive ordering of carbon in aromatic structures. Conversely, the increasing disorder trend is explained by the evolution of gas species forming throughout pyrolysis. The same peaks and their intensities are observed for the two particles sizes (1 and 3 mm), so particle size do not have any effect on the structural order in biochar.
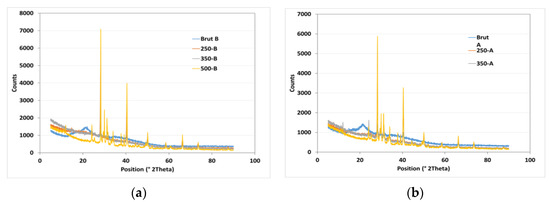
Figure 3.
XRD analysis of raw PB with a particle size of 1 mm (a) and 3 mm (b) and its derived biochar at temperatures of 250, 350, and 500 °C.
3.5. Scanning Electron Microscopy (SEM)
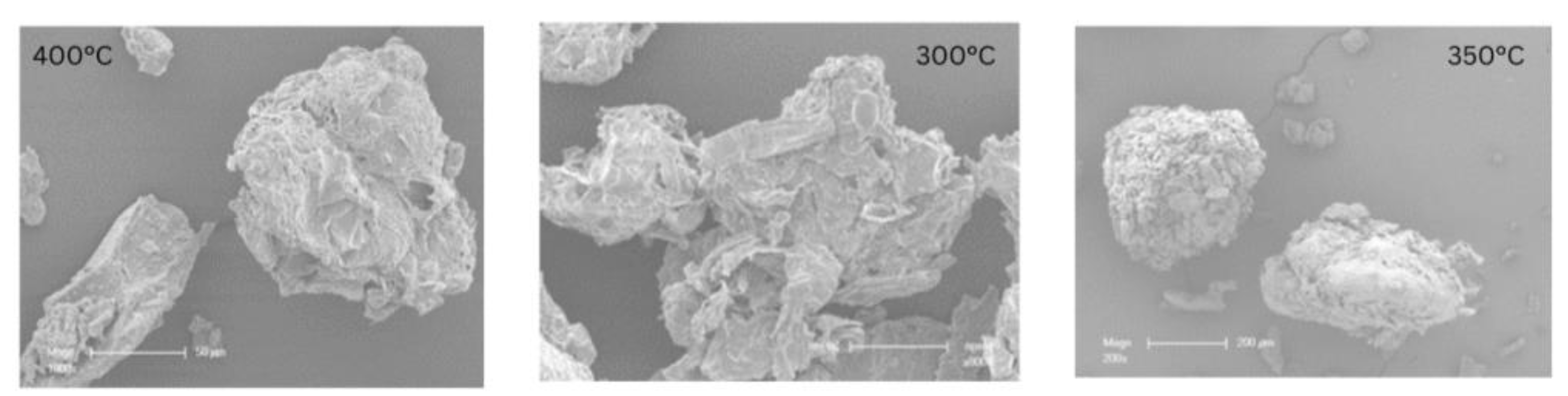
Figure 4 shows the SEM images of BP biochar produced at pyrolysis temperatures of 400, 300, and 350 °C, respectively. It can be seen that all biochar is composed of aggregates of different sizes. They have irregular shapes and structures.

Figure 4.
Scanning electron microscopy images of biochar at T = 300, 350 and 400 °C, magnified at ×1000.
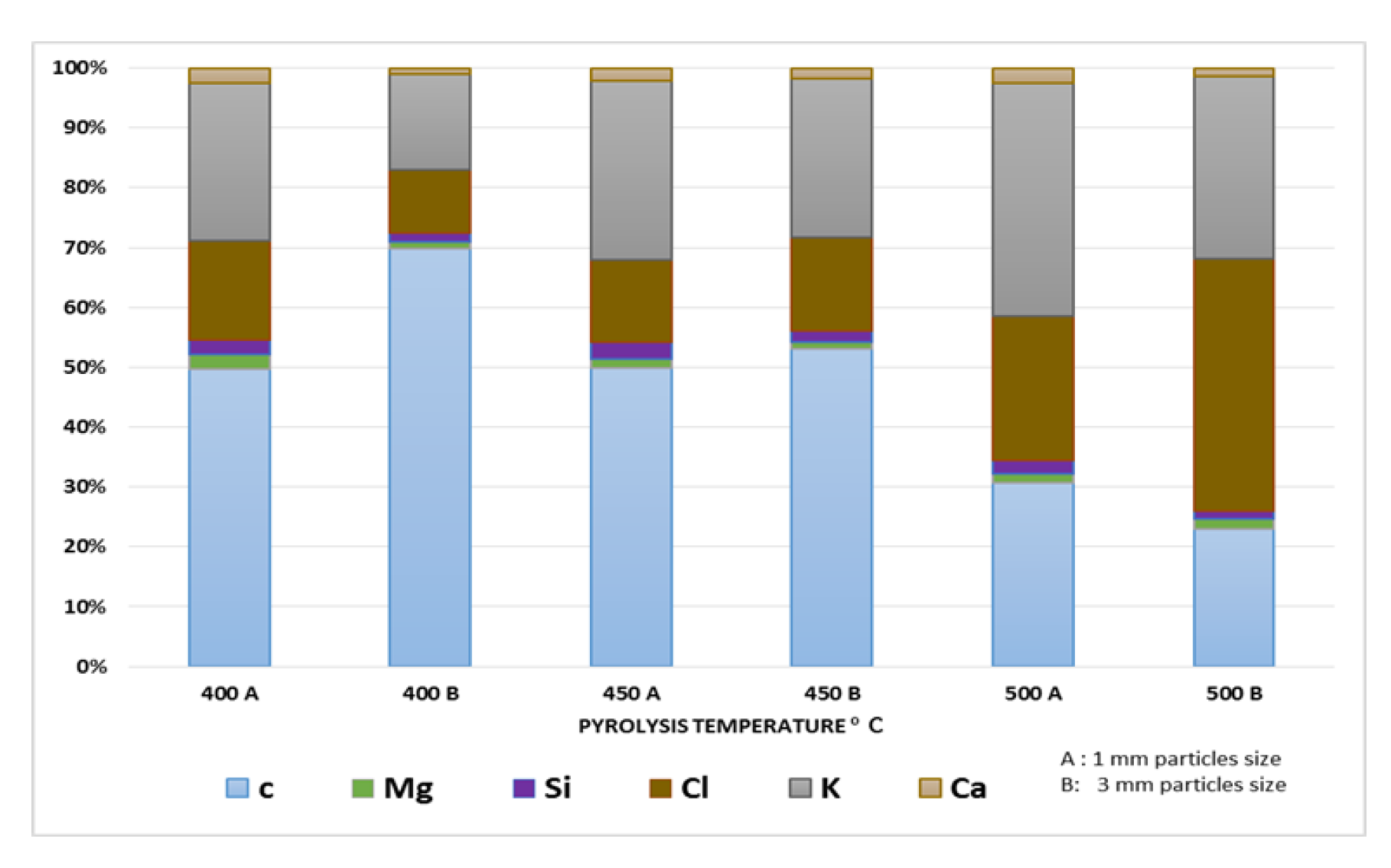
3.6. X-ray Fluorescence (XRF) Analysis
The composition of banana peel biochar (BPB) was analyzed, as shown in Figure 5. BPB contains mainly carbon, potassium, and chlorine. The percentage of these elements varied with pyrolysis temperature, and chlorine content was affected by particle size. Other minerals, such as calcium and magnesium, have been reported in varying levels in BPB due to differences in banana varieties, peel removal methods, and pyrolysis temperatures. The high percentage of chlorine in some banana peels may be due to pesticide contamination [13]. Further studies are needed to confirm the concentration and origin of chlorine in BPB and an elementary analysis should be conducted to determine the carbon and oxygen contents.
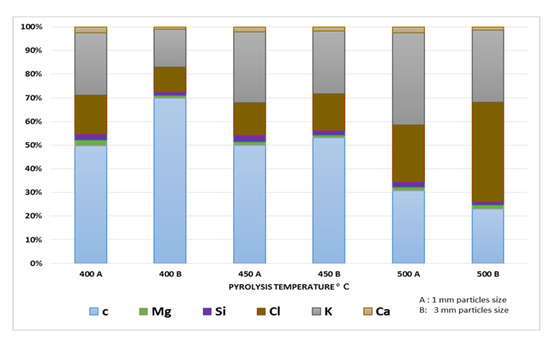
Figure 5.
Chemical composition of BPB at different pyrolysis T °C.
3.7. Copper Adsorption
In this study, the effect of different initial copper (Cu2+) concentrations on the adsorption capacity and removal efficiency of biochar was investigated. For this experiment, the pH was kept constant at 4.95 and a contact time of 60 min with an adsorbent dose of 0.5 g was used. The adsorption capacity was calculated using the following equation:
where qe, the equilibrium adsorption capacity (mg/g); C0 and Ce, the initial and equilibrium copper concentrations in the water (mg/L), respectively; V, volume of used water (L); and m, the mass of dried (grounded powder) bioadsorbent (g).
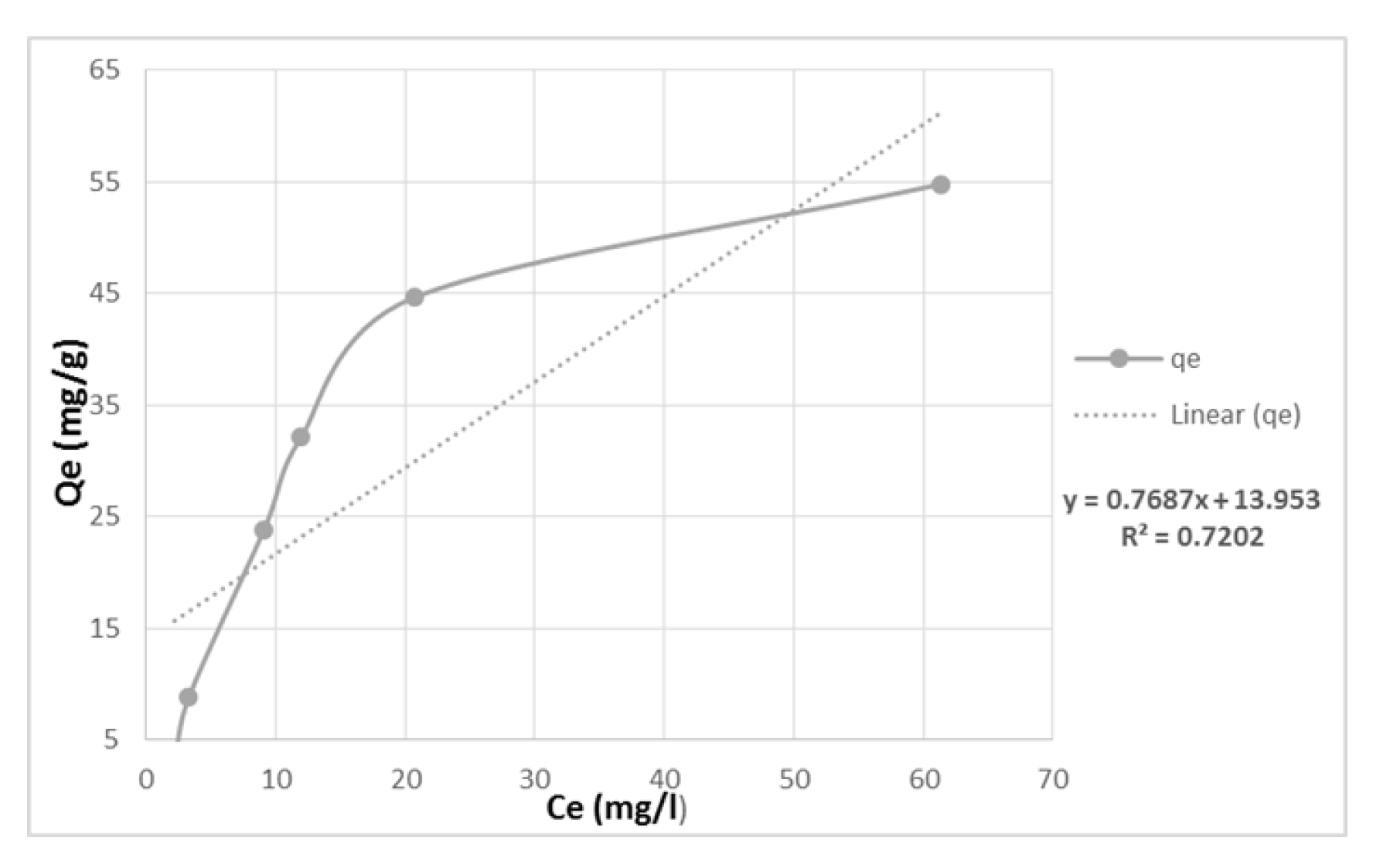
The results showed that the adsorption capacity increased with an increase in Cu2+ concentration from 50 to 550 mg/L and the removal efficiency also improved by approximately 40% for Cu2+ (Figure 6). The Langmuir model was found to be a better fit for the experimental data than the Freundlich model, indicating that the adsorption process is heterogenous on monomolecular layer surfaces through the chemical processes. The Freundlich constant ‘n’ value for Cu was 5.31, indicating a favorable adsorption process (Table 1). However, the Q max value was lower than the experimentally attained values, suggesting that the adsorbents may reach saturation capacity at lower initial metal concentrations.
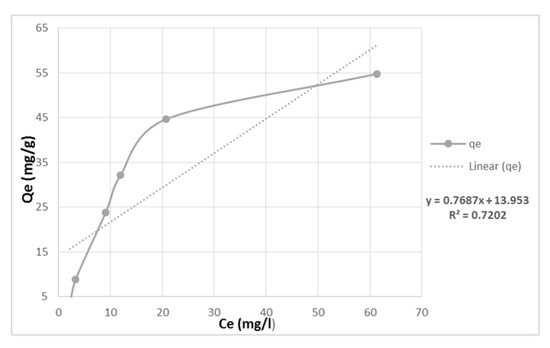
Figure 6.
Adsorbed quantity (Qe) versus the equilibrium copper concentrations in the water.

Table 1.
Freundlich and Langmuir model.
4. Conclusions
This study shows that banana peel can be a low-cost and effective source of biomass for producing biochar, which has great potential for removing heavy metals like copper from synthetic solutions. This study highlights the importance of pyrolysis conditions for optimizing biochar properties and further research is needed to evaluate its effectiveness for different applications and to optimize pyrolysis conditions. Overall, this study suggests that banana peel could be a promising resource for producing biochar, contributing to more sustainable production processes.
Author Contributions
G.K., M.I., M.J. and A.H. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sweep-Net. Country Report on the Solid Waste Management in Lebanon; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ): Bonn, Germany, 2014. [Google Scholar]
- Abbas, I.I.; Chaaban, J.K.; Al-Rabaa, A.R.; Shaar, A.A. Solid waste management in Lebanon: Challenges and recommendations. Environ. Sci. Pollut. Res. Int. 2017, 24, 8654–8664. [Google Scholar]
- Human Rights Watch. Lebanon: Huge Cost of Inaction on Trash Crisis. 2020. Available online: https://www.hrw.org/news/2020/06/09/lebanon-huge-cost-inaction-trash-crisis (accessed on 12 June 2020).
- Alam, P.; Ahmade, K. Impact of solid waste on health and the environment. J. Educ. Pract. 2013, 2, 23–29. [Google Scholar]
- Kabenge, I.; Omulo, G.; Banadda, N.; Seay, J.; Zziwa, A.; Kiggundu, N. Characterization of banana peel wastes as potential slow pyrolysis feedstock. J. Sustain. Dev. 2018, 11, 14. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit Peel Waste: Characterization and Its Potential Uses. Curr. Sci. 2017, 113, 444. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Qi, W.; Zhai, L.; Wang, F.; Zhang, J.; Li, D. Magnetic biochar is synthesized with waterworks sludge and sewage sludge and its potential for removing methylene blue. J. Environ. Chem. Eng. 2021, 9, 105951. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, R.; Xia, B.; Ying, R.; Hu, Z.; Tao, X.; Yu, H.; Xiao, F.; Chu, Q.; Chen, H.; et al. Effect of Pyrolysis Temperature on Removal Efficiency and Mechanisms of Hg(II), Cd(II), and Pb(II) by Maize Straw Biochar. Sustainability 2022, 14, 9022. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.U.; Jiang, F.; Guo, Z.; Peng, X. Does biochar application improve soil aggregation? A meta-analysis. Soil Tillage Res. 2021, 209, 104926. [Google Scholar] [CrossRef]
- Feitosa, A.A.; Teixeira, W.G.; Ritter, E.; Resende FA, D.; Kern, J. Characterization of biochar samples of banana peels and orange bagasse carbonized at 400 and 600 °C. Rev. Virtual Quim. 2020, 12, 901–912. [Google Scholar]
- Nasreddine, L.; Rehaime, M.; Kassaify, Z.; Rechmany, R.; Jaber, F. Dietary exposure to pesticide residues from foods of plant origin and drinks in Lebanon. Environ. Monit. Assess. 2016, 188, 485. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).