1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a widely used class of drugs that offer pain relief and reduce inflammation. Although they are not addictive, they can cause side effects such as an increased risk of heart attack, kidney disease, and dyspepsia. In addition, these drugs are highly capable of passing through biological membranes due to their high octanol–water partition coefficients (K
ow). Naproxen, diclofenac, and mefenamic acid are the most common NSAIDs and are also persistent pollutants that have attracted attention from scientists in recent decades. Therefore, it is important to measure drug concentrations in biological fluids for pharmaceutical analysis, clinical toxicology, and forensic tests [
1].
Preparing samples is an important process for detecting small amounts of substances in biological mixtures. Effective techniques should concentrate the desired substances and purify the samples while using minimal amounts of organic solvents and sample volume [
2]. Adequate preparation can enhance the sensitivity and compatibility of analytical instruments. Methods for extracting substances are advancing at a fast pace, along with improvements in analytical techniques.
In the past few years, there has been a focus on developing environmentally friendly sample preparation methods. Although liquid phase microextraction (LPME) is common for determining analytes, it requires a considerable amount of toxic solvents. Electromembrane extraction (EME) is an alternative that extracts ionized compounds from complex matrices with minimal organic solvent use. This process works by applying an electric potential across a membrane, causing analytes to migrate through a supported liquid membrane (SLM) into an aqueous solution. The efficiency of the extraction depends on several factors, such as the pH, donor and acceptor phase compositions, electric field magnitude, and type of membrane [
3,
4]. EME offers advantages such as fast analysis, simplicity, sensitivity, adaptability, and excellent clean-up ability.
To improve the accuracy of ultra-trace analysis using EME, a combination of two extraction methods can be used to increase the enrichment and decrease the limit of detection [
5]. By combining EME with a technique that deals with the complexity of the sample matrix, it is possible to achieve effective sample clean-up and reduce the amount of organic solvent required.
In the current study, a new method combining EME and solid phase microextraction (SPME) was employed to improve extraction efficiencies. Accordingly, a monolithic nanocomposite of poly(methacrylic acid-ethylene glycol dimethacrylate)/Cu-Cr layered double hydroxide (poly(MAA-EGDMA)/Cu-Cr LDH) was synthesized in the acceptor phase. This system was employed to determine naproxen, diclofenac, and mefenamic acid in human biological samples such as urine, plasma, and breast milk using high-performance liquid chromatography (HPLC).
2. Methods
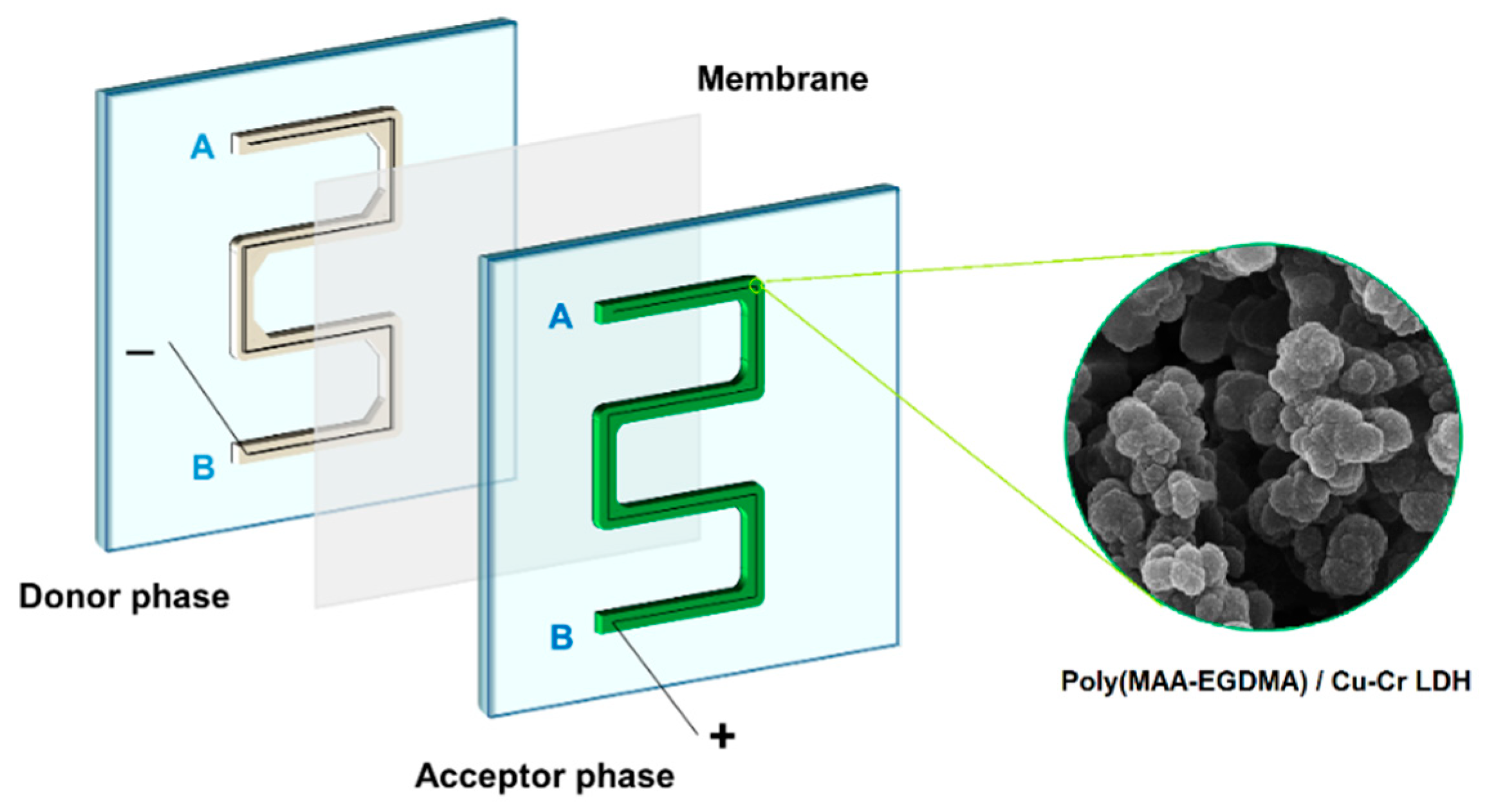
2.1. Fabrication of Microfluidic Device
The microfluidic chip for the EME-SPME technique was made from poly(methyl methacrylate) (PMMA) and had two separate symmetrical plates with the same channels. One of the channels was used as the acceptor phase where the composite was synthesized, while the other was used as the donor phase. Then, electrodes were inserted into each phase. A piece of polypropylene membrane was impregnated with SLM and placed between the two phases (
Figure 1). The chip was sealed with clamps for each extraction.
2.2. Poly(MAA-EGDMA)/Cu-Cr LDH Synthesis
Cu-Cr LDH was synthesized by dissolving metal nitrate salts (Cu(NO3)2·3H2O and Cr(NO3)3·9H2O) in ultra-pure water with a Cu2+/Cr3+ ratio of 3:1 and adjusting the acidity to pH = 9 by gradually adding NaOH solution (2.0 M). Then, the resulting slurry was aged for 24 h, washed, and dried in a vacuum oven to obtain a green powder.
To synthesize poly(MAA-EGDMA), a pre-polymerization mixture containing methacrylic acid (MAA) monomer, ethylene glycol dimethacrylate (EGDMA) as a cross-linker, toluene, dodecanol as the porogenic solvent, and 2,2′-azobis(2-methylpropionitrile) (AIBN) as an initiator was prepared. Cu-Cr LDH was added to the mixture and sonicated, and the mixture was purged with nitrogen before undergoing pre-polymerization at 75 °C in a water bath. The gel formed during pre-polymerization was injected into the acceptor phase channel of the microfluidic chip, and polymerization was carried out at 60 °C for 16 h. The composite was washed with methanol to remove unreacted components and excessive porogenic solvents.
2.3. The Extraction Procedure
To start the extraction, a piece of the membrane was impregnated with 1-octanol and placed between the donor and acceptor phases of the microfluidic chip. A specific voltage (optimum value of 15 V) was applied while the sample solution was pumped into the donor channel inlet. After the target analytes were absorbed into the sorbent, the desorption solvent was passed through the sorbent and into the acceptor phase. The collected desorption solvent was then analyzed via HPLC. After each extraction, the sorbent was washed and the membrane was replaced.
3. Results and Discussion
3.1. Optimization of Effective Parameters
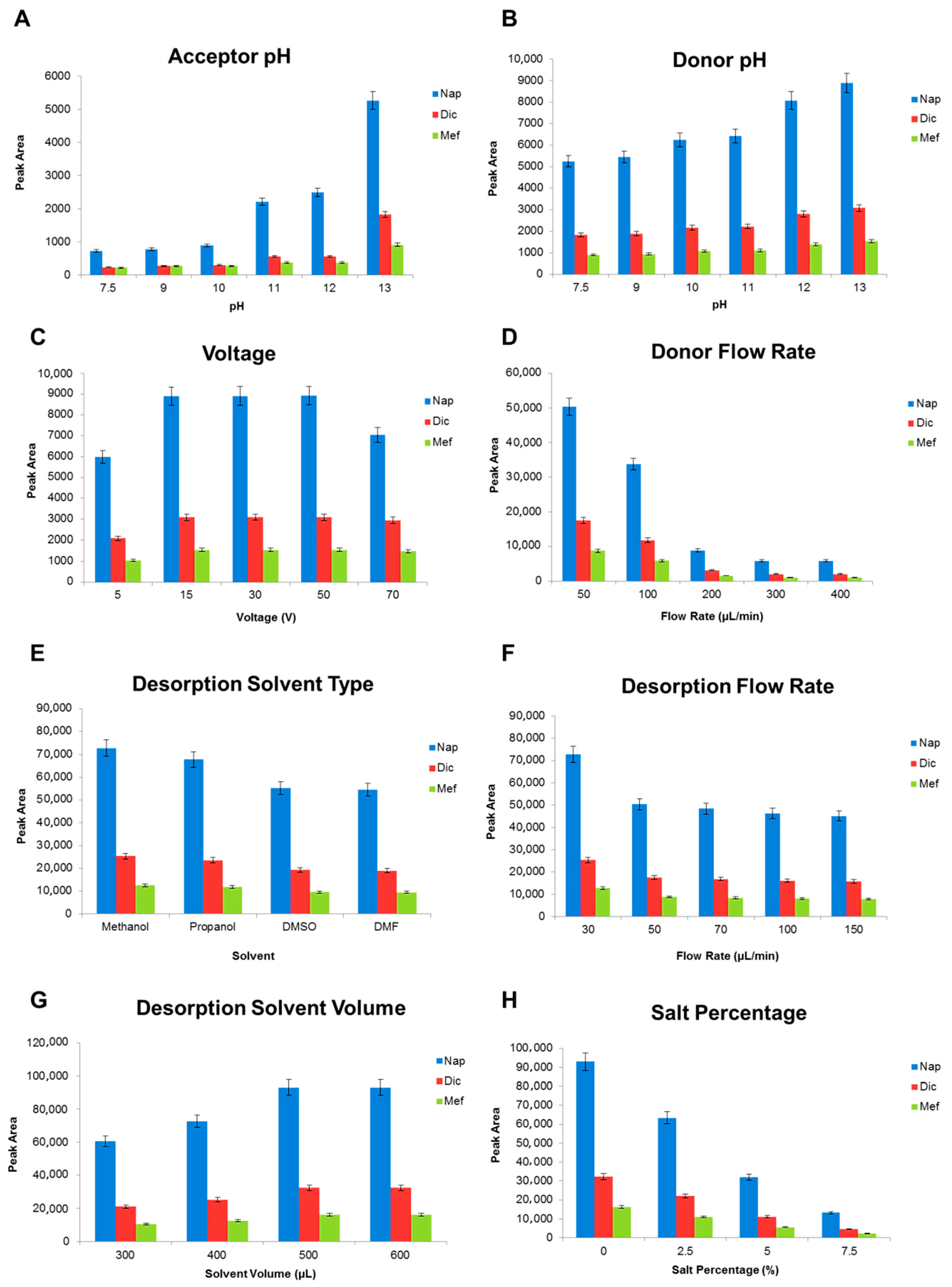
To obtain the best possible results for the extraction of the model analytes, the effective parameters with relation to extraction were optimized using one variable at a time. The optimization process involved adjusting various parameters such as the pH of the acceptor and donor, voltage, flow rate of sample and desorption solution, type and volume of desorption solvent, and salt percentage. Consequently, the optimized parameters were selected for the rest of the experiments, and the relevant diagrams are shown in
Figure 2. Accordingly, extraction efficiencies increased from 5 to 15 V, and then remained consistent. Within the potential over 50 V, extraction efficiencies decreased which can be due to the electrolysis of water and back extraction (because of the decrease in pH). Moreover, on account of the instability caused by the Joule heating effect (electric current more than 100 mA and the evaporation of 1-octanol), 15 V was selected for further experiments [
6].
3.2. The Extraction Procedure
The figures of merit for each analyte were evaluated by plotting calibration curves in an aqueous sample solution under optimized conditions. The linear dynamic ranges achieved were 0.5–500 μg L
−1 for naproxen and 1.00–250 μg L
−1 for diclofenac and mefenamic acid (R
2 ≥ 0.9962). The method was found to be appropriate for determining low concentrations of the compounds based on the LODs of 0.10–0.25 μg L
−1. Additionally, preconcentration factors and extraction recoveries were sufficient (
Table 1).
3.3. Analysis of Real Samples
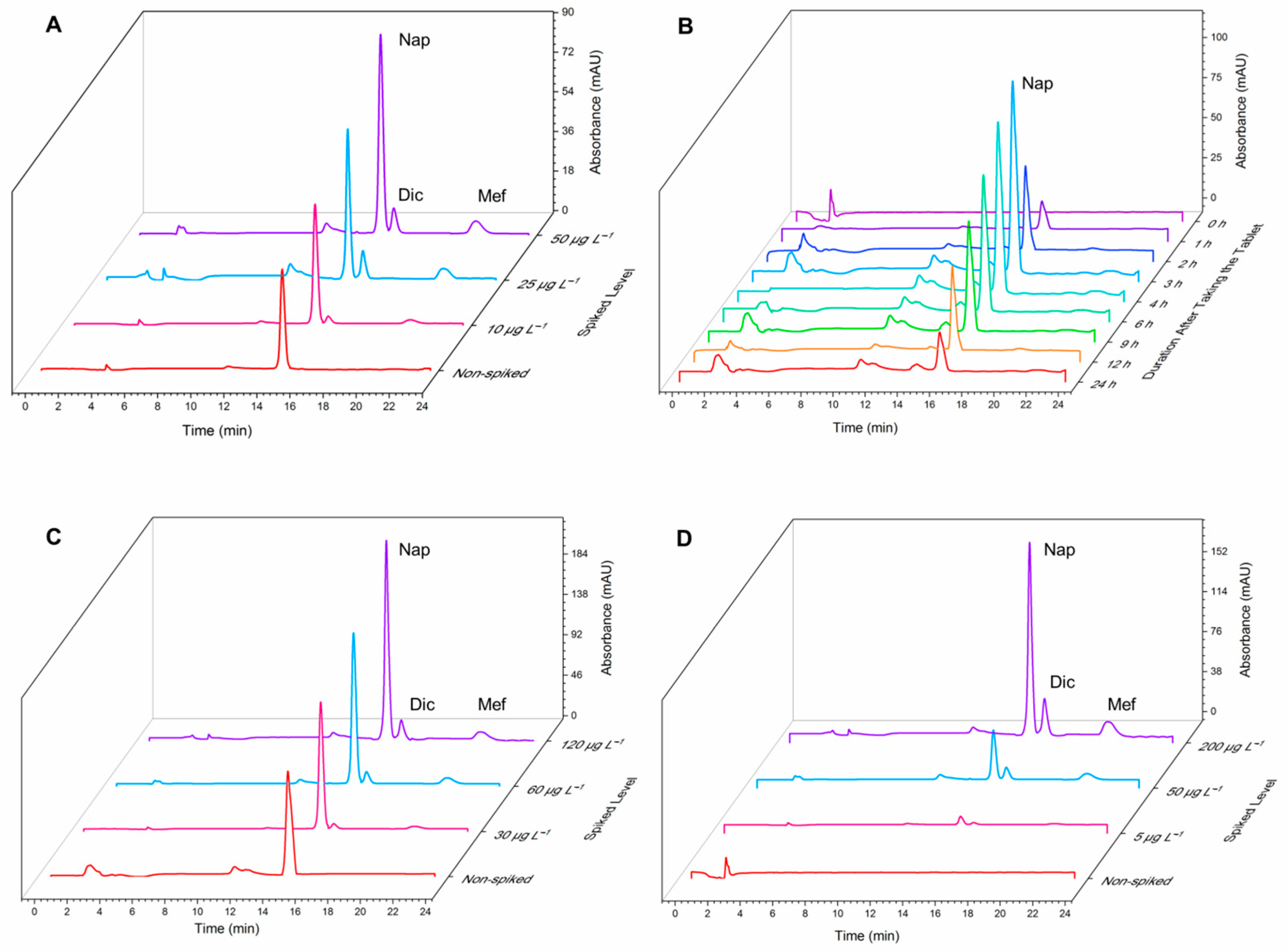
Eventually, EME-SPME’s capability for the determination of NSAIDs in urine, breast milk, and plasma samples was investigated. The method was successful in detecting naproxen in breast milk and urine samples, but none of the target analytes were found in the plasma sample. The accuracy of the method was assessed by spiking the samples with different concentration levels of standard drugs, and satisfactory relative recoveries were obtained. The chromatograms used to support the findings are provided in
Figure 3.
4. Conclusions
This work introduced a novel combination of EME and SPME methods using a microfabricated device to increase extraction efficiency and applicability for the extraction of naproxen, diclofenac, and mefenamic acid from complex sample matrices. Poly(MAA-EGDMA)/Cu-Cr LDH was synthesized in the acceptor phase channel and was found to be a suitable solid phase due to its monolithic structure and porosity. The method showed higher efficiency than the conventional method, and the microfluidic device provided high clean-up, sensitivity, accuracy, and low organic solvent consumption for determining model drugs in biological samples.
Author Contributions
Y.Y.: inception of the project design, method development, and supervision. R.Z.: conceptualization, methodology, experimentation, data acquisition, in addition to writing the original draft prepared. All authors have read and agreed to the published version of the manuscript.
Funding
This research acknowledges funding from Tarbiat Modares University, Tehran, Iran in the discovery grant category.
Institutional Review Board Statement
The study was approved by the Iran International Committee for Ethics in Biomedical Research with the approval no. of IR.MODARES.REC.1401.016.
Informed Consent Statement
In regard to sampling, informed consent was obtained from all the involved volunteers before entering the study.
Data Availability Statement
All the relevant data have been included in the manuscript.
Acknowledgments
Nasser Nikfarjam and Payam Osouli are gratefully thanked for their support and constructive scientific consultation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Almeida, H.F.D.; Marrucho, I.M.; Freire, M.G. Removal of Nonsteroidal Anti-Inflammatory Drugs from Aqueous Environments with Reusable Ionic-Liquid-Based Systems. ACS Sustain. Chem. Eng. 2017, 5, 2428–2436. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Rociek, A.; Cieślik, E.; Sieja, K. Simultaneous Sample Preparation Method for Determination of 3-Monochloropropane-1,2-Diol and Polycyclic Aromatic Hydrocarbons in Different Foodstuffs. Food Anal. Methods 2016, 9, 2906–2916. [Google Scholar] [CrossRef]
- Hasheminasab, K.S.; Fakhari, A.R.; Shahsavani, A.; Ahmar, H. A new method for the enhancement of electromembrane extraction efficiency using carbon nanotube reinforced hollow fiber for the determination of acidic drugs in spiked plasma, urine, breast milk and wastewater samples. J. Chromatogr. A 2013, 1285, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rye, T.K.; Martinovic, G.; Eie, L.V.; Hansen, F.A.; Halvorsen, T.G.; Pedersen-Bjergaard, S. Electromembrane extraction of peptides using deep eutectic solvents as liquid membrane. Anal. Chim. Acta 2021, 1175, 338717. [Google Scholar] [CrossRef] [PubMed]
- Ramin, M.; Omidi, F.; Khadem, M.; Shahtaheri, S.J. Combination of dispersive solid-phase extraction with dispersive liquid-liquid microextraction followed by high-performance liquid chromatography for trace determination of chlorpyrifos in urine samples. Int. J. Environ. Anal. Chem. 2019, 101, 810–820. [Google Scholar] [CrossRef]
- Kjelsen, I.J.Ø.; Gjelstad, A.; Rasmussen, K.E.; Pedersen-Bjergaard, S. Low-voltage electromembrane extraction of basic drugs from biological samples. J. Chromatogr. A 2008, 1180, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).