Abstract
The contamination of water and food with antibiotics residues poses a severe risk to human health and aquatic environments. The excessive and uncontrolled use of antibiotics is one of the major causes of their presence in the environment. Their continuous consumption willingly or un-willingly can result in severe health issues such as allergy, headache, hypertension, muscle pain, and hormonal dysfunction. Beside these, the development of antimicrobial resistance (AMR) can make the situation more critical. Therefore, advanced analytical approaches over conventional techniques are required to detect antibiotic residues in a facile and cost-effective manner. The present work deals with the design of fluorescent nanostructures as sensing probes for the detection of ciprofloxacin. Here, we have synthesized NH2-MIL-53(Al) using a hydrothermal approach. This fluorescent metal–organic framework (MOF) was further combined with molecular imprinted polymers (MIPs) for the selective and specific detection of ciprofloxacin in aqueous solutions. The use of MIPs over other biomolecules (such as antibody, enzymes, and others) is highly promising as it avoids any kind of pre-treatment of the sample. The MIP@NH2-MIL-53(Al) nanostructure formation is confirmed by performing different characterization techniques involving both spectroscopy and microscopy. The performance of the developed fluorescent composite promotes its applicability for the highly sensitive and specific detection of ciprofloxacin in practical applications.
1. Introduction
All over the world, the emergence of antibiotics in the environment has been recognized as a serious threat to both humans and the ecosystem. Their accumulation in water resources can lead to several health issues and endanger aquatic life. Prolonged exposure to antibiotics in the environment can cause the development antimicrobial resistance (AMR). In such a condition, the treatment of simple bacterial infections will no longer be possible. As per the World Health Organization (WHO) report [1], AMR is among the top global major public health threats that require urgent multisectoral action. Therefore, it is highly desirable to develop facile strategies for monitoring the presence of antibiotics in aqueous solutions. Among different antibiotics, ciprofloxacin (with broad-spectrum antibacterial activity and lower side effects) is extensively used to treat urinary tract infections, lung infections, cell carcinoma, and cystic fibrosis [2,3]. However, unintentional exposure and further accumulation of this in the human body can cause serious health issues such as hematuria, gastrointestinal complaints, skin reactions, and liver damage [4,5]. Among the different conventional techniques such as chromatography, spectrofluorimetry, electrophoresis, electrochemical, etc., the fluorescence approach is a promising method due to its ease of operation, quick response, low cost, and high sensitivity [6,7,8].
Nowadays, different fluorescent materials, especially metal–organic frameworks (MOFs), have been developed to replace the fluorescent dyes. MOFs offer several unique photophysical properties such as large surface area, structure tunability, photostability, high porosity, and narrow fluorescence emission. Several research reports explored using fluorescent MOFs for the detection of ciprofloxacin. For instance, An et al. [9] reported the Tb-based coordination polymer (CP) for the fluorescent detection of ciprofloxacin. Here, ciprofloxacin offered a sensitization effect on the luminescence of Tb3+. Beside water and milk samples, the Tb-based complexes have also been reported for ciprofloxacin detection in urine samples [10]. However, the toxic nature of lanthanides can limit their application for real-field monitoring. Liu et al. reported the Zr-based MOF functionalized with citrate (C-MOF-808) for the fluorescent detection of ciprofloxacin in aqueous solutions [11]. Beside the high sensitivity of these fluorescent sensors, their combination with molecularly imprinted polymers (MIPs) can offer specific target detection capabilities [12,13]. The incorporation of MIPs into porous MOFs can support the excellent selective detection capabilities. The high surface area and availability of abundant pores in MOFs is highly favorable for the synthesis of imprinting sites. However, there are very few examples in the literature on the use of this combination of MOFs and MIPs for the detection of antibiotics. Herein, Al-based MOFs were developed first via a hydrothermal approach and later were combined with ciprofloxacin-specific MIPs. The fluorescent properties of both NH2-MIL-53(Al) and its composite with MIPs were examined for the specific detection of ciprofloxacin.
2. Materials and Method
2.1. Materials
The aluminium(III) chloride hexahydrate (AlCl3·6H2O), N,N-dimethylformamide (DMF), 2-aminoterephthalic acid (NH2-BDC), and ciprofloxacin were purchased from Sigma-Aldrich (St. Louis, MO, USA). The urea, tetraethoxysilane (TEOS), aminopropltriethoxysilane (APTES), and ethanol were procured from HiMedia Pvt. Ltd. (Bengaluru, India). The ammonia solution was purchased from E. Merck Ltd. (Darmstadt, Germany). All these analytical grade chemicals were utilized as received without any kind of purification. The distilled water (DW) was prepared in a laboratory using distillation unit.
2.2. Synthesis of NH2-MIL-53(Al)
The Al-based MOF was synthesized via a hydrothermal approach, as earlier reported in [14]. In detail, 0.3 M AlCl3·6H2O was dissolved in 10 mL DW and was mixed in a NH2-BDC solution (0.2 M, 15 mL DMF) under continuous stirring. After 10 min, 5 mL aqueous solution of urea (1 M) was dropped in the above solution and the mixture was stirred for another 10–15 min. Thereafter, the solution was poured into Teflon-lined autoclave and heated to 150 °C for 6 h. The collection of the resulting yellow precipitates was performed with the help of centrifugation at 9000 rpm for 15 min and they were further washed with DW. For activation, the synthesized product was redispersed in 20 mL methanol and DMF solution under dark stirring overnight. The final produce was centrifuged and dried at 80 °C. The resultant product was collected for further characterization.
2.3. Synthesis of NH2-MIL-53(Al)/MIP
The NH2-MIL-53(Al)/MIP nanocomposite was prepared using a sol–gel approach [15,16]. Briefly, the aqueous solution of the above synthesized MOF (30 mg) and ethanol (10 mL) were added in a reagent bottle. Then, 0.1 mL APTES was added in the above solution under magnetic stirring for self-assembly of the APTES over the MOF structure. Further, a template ciprofloxacin solution (1 mg/mL) was added in the above solution and allowed to stir for 20 min. Next, a drop of ammonia hydroxide was added, followed by dropwise addition of TEOS (1 mL) and ethanol (10 mL). After overnight stirring of this reaction mixture at room temperature, the final product was collected via centrifugation. The product was washed thoroughly with DW and ethanol in subsequent steps up to five times. The final product was dried and stored for further characterization.
2.4. Fluorescence Detection of Ciprofloxacin
In this experiment, the PL of the synthesized material (0.02 mg·mL−1) was examined with varied excitation from 290 to 390 nm. The emission was recorded between 350 and 450 nm at a fixed excitation of 330 nm. The PL of the materials was also studied in the presence of ciprofloxacin (100 µM and 1000 µM) with a 3:1 ratio of ciprofloxacin and MOF or MOF/MIP.
2.5. Characterization of NH2-MIL-53(Al) and NH2-MIL-53(Al)/MIP
The crystalline structure of NH2-MIL-53(Al) was studied using an X-ray diffractometer (Rigaku Ultima diffractometer, USA). The analysis of the porosity and surface area of synthesized MOF was performed with the help of Belsorp Max system (Microtrac, Osaka, Japan). The hydrodynamic diameter and zeta potential of the synthesized structure were also analyzed using a Zeta Sizer (Malvern Instruments, Malvern, UK). The UV-Vis absorption characteristics of NH2-MIL-53(Al) and its composite with MIP were recorded using a Shimadzu UV-3600 spectrometer. The functional groups over the surface of the synthesized materials were studied using Fourier transform infrared (FTIR; Perkin Elmer, Waltham, MA, USA) spectroscopy. The morphology and topography of the materials were examined using field emission-scanning electron microscopy (FESEM; JSM6100, Jeol, Akishima, Tokyo) along with energy-dispersive X-ray (EDX) spectroscopy. The fluorescence spectra of the materials were recorded using a SpectraMax® system (Molecular Devices, San Jose, CA, USA).
3. Results and Discussion
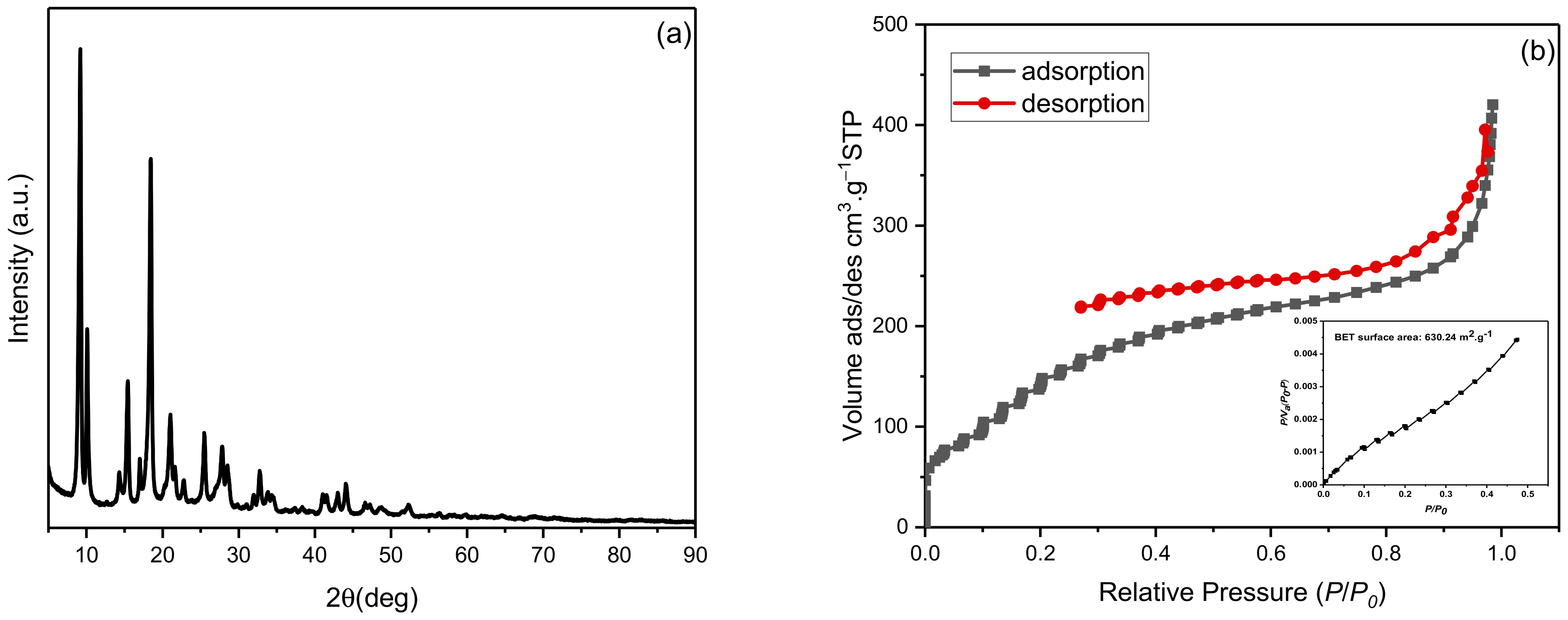
The synthesized NH2-MIL-53(Al) is well crystallized as confirmed using the XRD pattern, Figure 1a. All the prominent characteristic peaks are in accordance with [17]. Using the Debye Scherrer equation, the crystallize size of NH2-MIL-53(Al) was calculated as 24.34 nm with a lattice strain of 0.0186. The N2 adsorption/desorption isotherms of the synthesized MOF are presented in Figure 1b. The surface area of the MOF using the BET method was found to be 630.24 m2·g−1 along with an average pore diameter of 4.126 nm and a total pore volume of 0.6501 cm3·g−1. The hydrodynamic diameter of the MOF was found to be ~483 nm with a polydispersity index of 0.573. The highly positive zeta potential (20.2 mV) of NH2-MIL-53(Al) confirms its aqueous stability, which tends to avoid agglomeration.
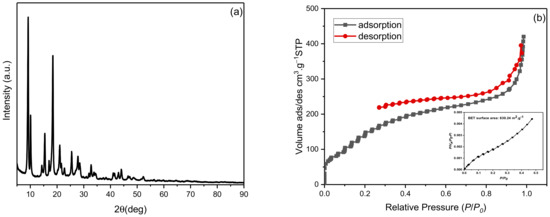
Figure 1.
Characterization of synthesized NH2-MIL-53(Al): (a) XRD pattern and (b) Nitrogen adsorption/desorption isotherms (inset: BET plot) from N2 isotherm at 77.350 K.
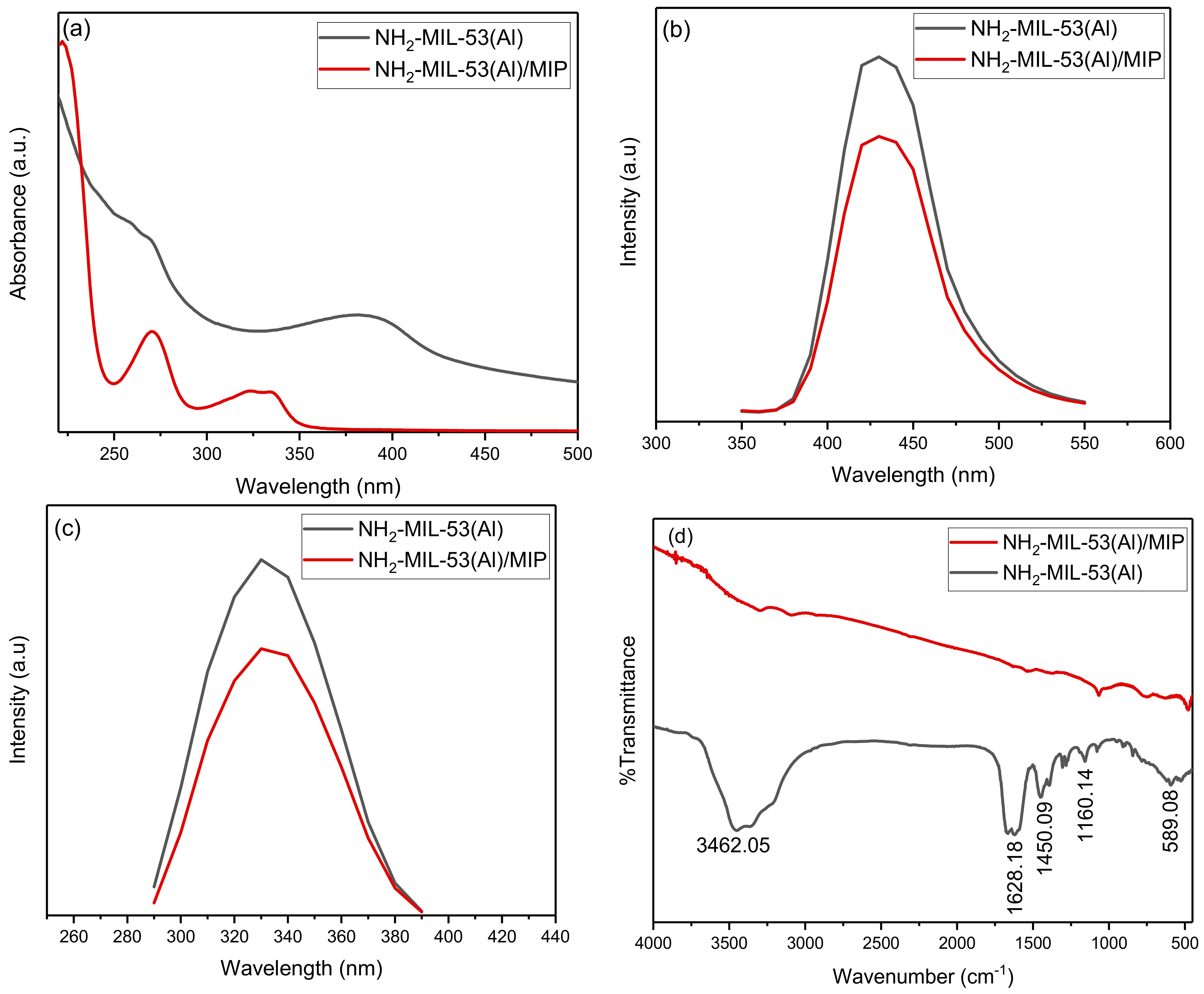
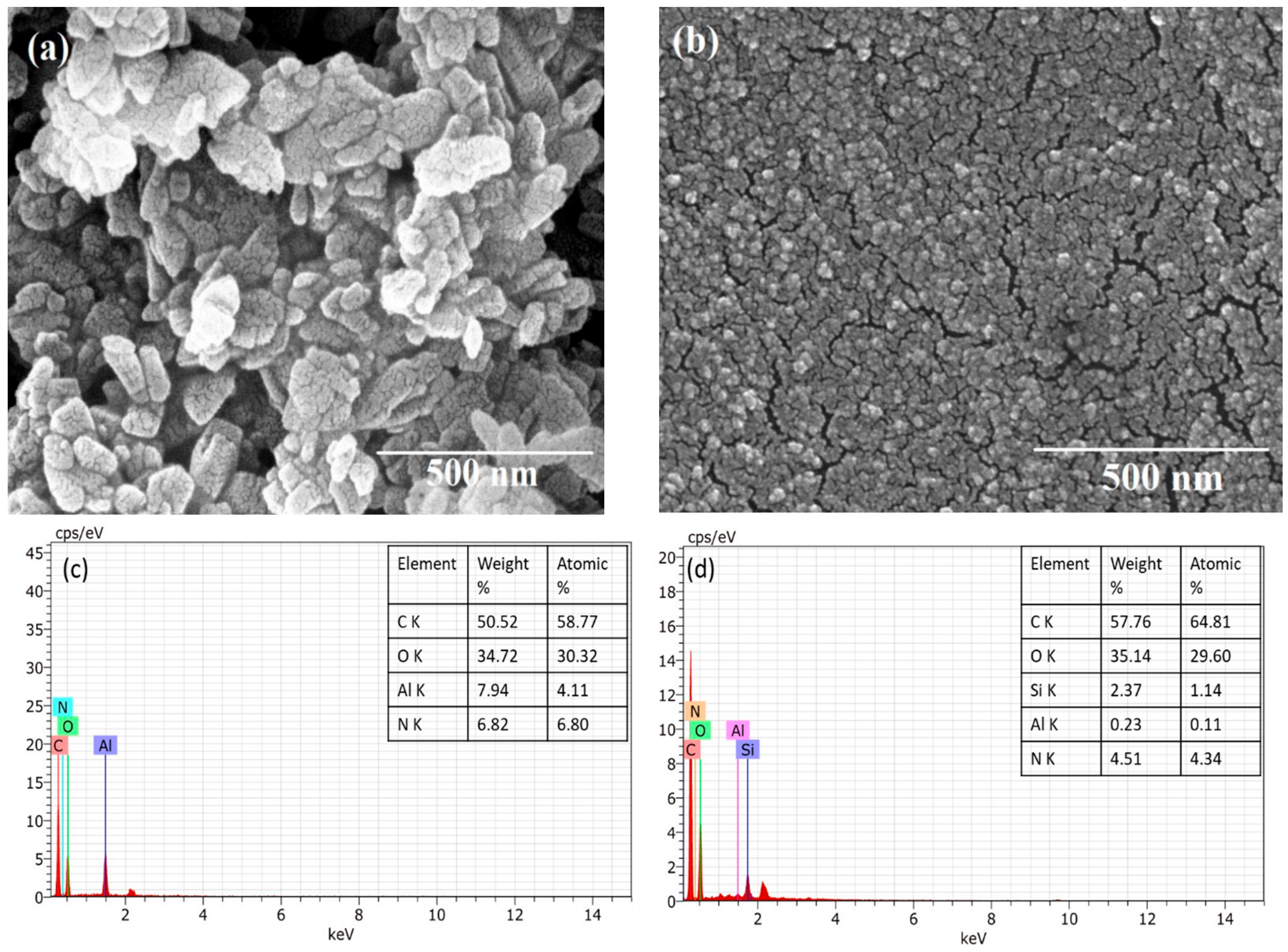
In the UV-Vis spectra (Figure 2a), two absorption peaks were found for NH2-MIL-53(Al) at 270 and 380 nm. The absorption peak at 380 nm changes to 330 nm after the interaction of NH2-MIL-53(Al) with the MIPs. Figure 2b shows the fluorescence spectra of both compounds at a fixed excitation of 330 nm. The interaction of MIPs with the MOF structure results in the small quenching of the fluorescence of MOF. The fluorescence of both compounds is also studied as a function of the varied excitation (refer to Figure 2c). In the FTIR spectra (Figure 2d), the broad adsorption band centered at 3462.05 cm−1 confirms the presence of the OH group in the framework. The peak at 1628.18 cm−1 can be allotted to N–H bending [18]. The typical vibration, which was due to the benzene linker, was observed at 1450.09 cm−1 and can be attributed to the aromatic ring stretch amines. A significant impact of the MIPs can be seen over the presence of functional groups of MOF. The size of as synthesized MOF was found within the 100 nm range with the shape symmetry (refer to Figure 3a). The uniform distribution of the MOF with MIP can be seen in Figure 3b. The EDS spectra of both compounds are presented in Figure 3c,d.
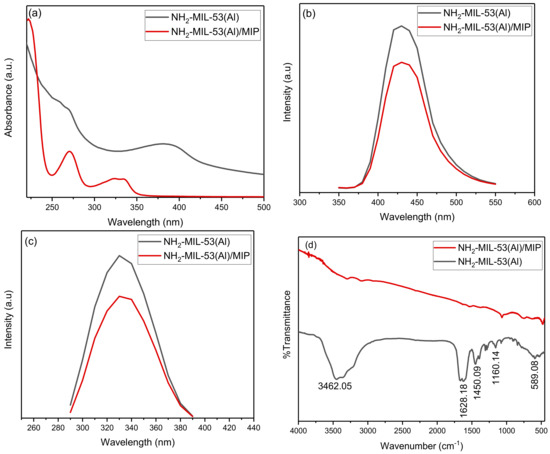
Figure 2.
Optical characteristics of NH2-MIL-53(Al) and NH2-MIL-53(Al)/MIP: (a) UV-Vis absorption, (b) fluorescence emission spectra at fixed excitation of 330 nm, (c) fluorescence emission spectra with varied excitation and fixed emission at 430 nm, and (d) FTIR spectra.
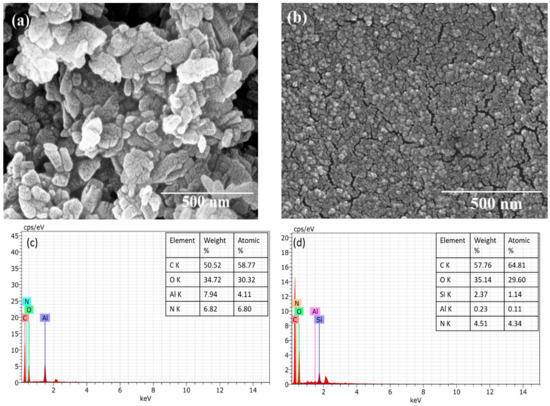
Figure 3.
FE-SEM images and EDX spectra of (a,c) NH2-MIL-53(Al) and (b,d) NH2-MIL-53(Al)/MIP, respectively.
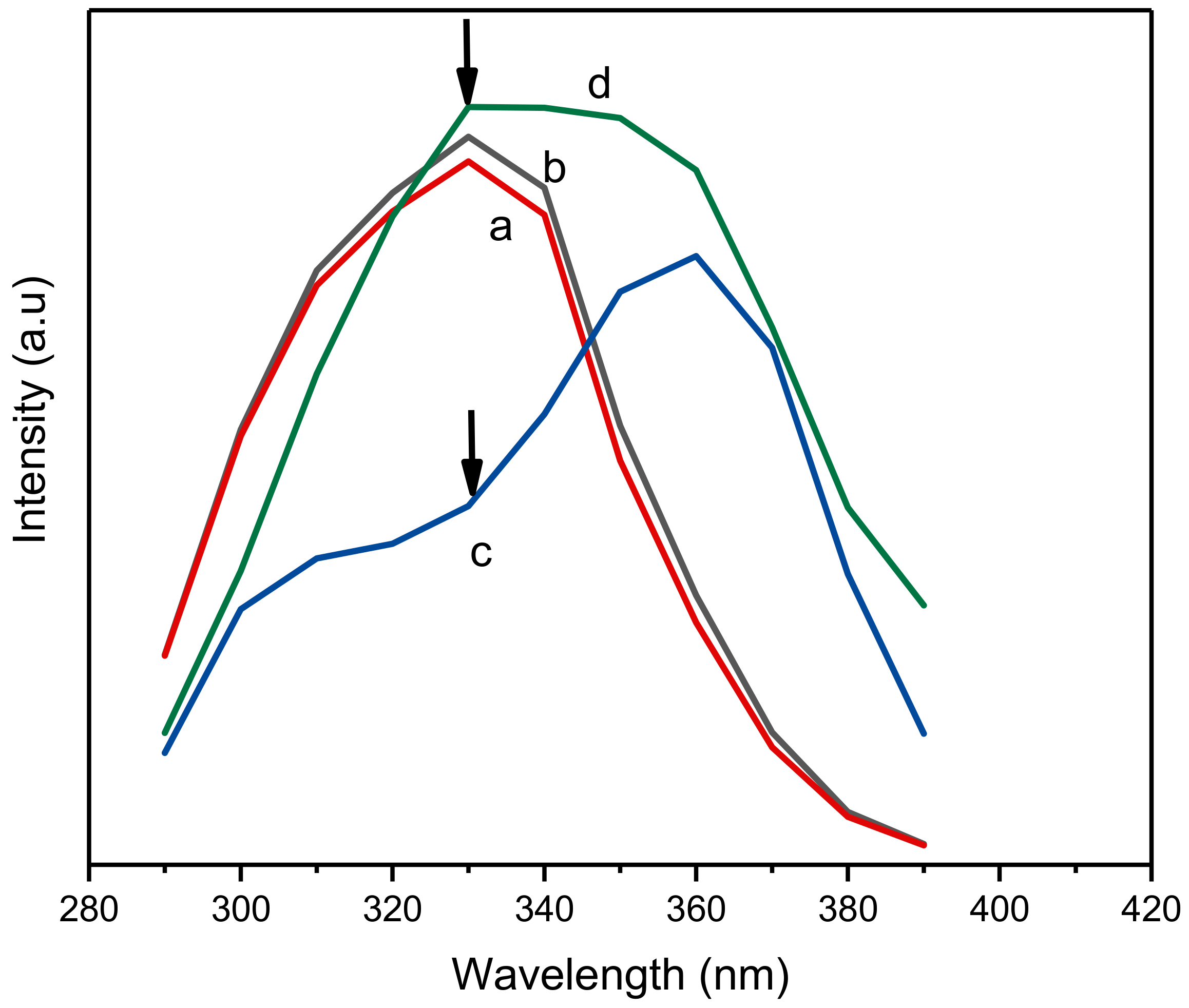
The effect of the concentration of ciprofloxacin on the fluorescence properties of Al-based MOF and its composite with MIPs is shown in Figure 4. Here, it is clear that there is no significant change in the fluorescence properties of NH2-MIL-53(Al) at lower (100 µM) and higher concentrations (1000 µM) of ciprofloxacin (refer to curve a and b, respectively). However, a significant change in the fluorescence of NH2-MIL-53(Al)/MIP can be observed with the increasing concentration of ciprofloxacin. As the concentration of ciprofloxacin increases from 100 µM to 1000 µM, a significant increase in the fluorescence emission at 430 nm was observed with an excitation of 330 nm (refer to curve c and d, respectively). This significant change in the fluorescence emission of the composite can be potentially utilized for the detection of ciprofloxacin.
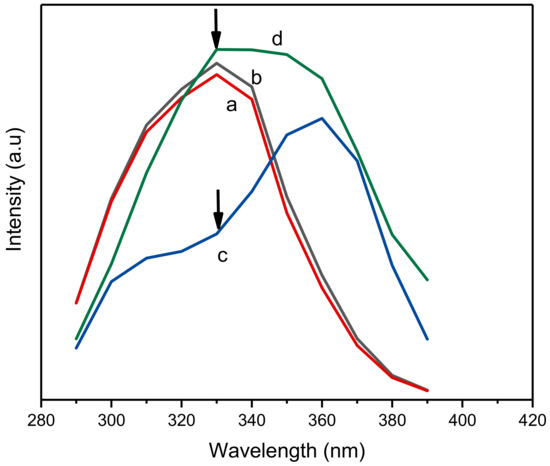
Figure 4.
Optical characteristics of NH2-MIL-53(Al) and NH2-MIL-53(Al)/MIP in the presence of different concentrations of ciprofloxacin where a and b denote NH2-MIL-53(Al) and c and d denote NH2-MIL-53(Al) in the presence of increasing concentration of ciprofloxacin (100 µM and 1000 µM, respectively). Here, arrows show the comparative change in PL intensity for c and d.
4. Conclusions
Nowadays, the screening of antibiotics is of utmost importance for the safety of ecosystems and human health. Here, the synthesis of fluorescent NH2-MIL-53(Al) was carried out using a hydrothermal approach. After confirmation of its crystalline nature and high surface area, its composite with MIP was developed via a sol–gel approach. Ciprofloxacin-specific MIP has a significant impact on the optical properties of NH2-MIL-53(Al), i.e., UV-vis absorbance and fluorescence properties. The significant change in the fluorescence emission from NH2-MIL-53(Al)/MIP (at 430 nm with an excitation at 330 nm) with the increasing concentration of ciprofloxacin can be effectively utilized for its detection.
Author Contributions
Conceptualization, S.K., R.K. and M.N.; methodology, M.N., A.R. and N.B.; validation, N.D., S.K. and M.N.; formal analysis, M.N., A.R. and N.B.; investigation, M.N. and R.K.; resources, S.K., R.K. and N.D.; data curation, M.N. and N.D.; writing—original draft preparation, M.N. and R.K.; writing—review and editing, R.K., S.K. and M.N.; supervision, R.K., N.D. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Monika Nehra is grateful to CSIR for the SRA fellowship (No. B-12857 Dated 21 October 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 19 March 2023).
- Tyulenev, A.V.; Smirnova, G.V.; Muzyka, N.G.; Oktyabrsky, O.N. Study of the early response of Escherichia coli lpcA and ompF mutants to ciprofloxacin. Res. Microbiol. 2022, 173, 103954. [Google Scholar] [CrossRef] [PubMed]
- Watcharadulyarat, N.; Rattanatayarom, M.; Ruangsawasdi, N.; Patikarnmonthon, N. PEG–PLGA nanoparticles for encapsulating ciprofloxacin. Sci. Rep. 2023, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Quan, H.; Yin, S.; Sun, L.; Lu, H. Unraveling the Toxicity Associated with Ciprofloxacin Biodegradation in Biological Wastewater Treatment. Environ. Sci. Technol. 2022, 56, 15941–15952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, H.; Duan, A.; Wang, Y. Mechanistic insight into the degradation of ciprofloxacin in water by hydroxyl radicals. J. Hazard. Mater. 2023, 446, 130676. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, T.; Lu, Y.; Wang, W.; Zhou, Z.; Yan, Y. Constructing carbon dots and CdTe quantum dots multi-functional composites for ultrasensitive sensing and rapid degrading ciprofloxacin. Sens. Actuators Chem. 2019, 289, 242–251. [Google Scholar] [CrossRef]
- Munir, F.; Waseem, M.T.; Khan, Z.A.; Majeed, S.; Farooq, U.; Shahzad, S.A. Synthesis of AIEE active triazine based new fluorescent and colorimetric probes: A reversible mechanochromism and sequential detection of picric acid and ciprofloxacin. J. Photochem. Photobiol. Chem. 2022, 429, 113921. [Google Scholar] [CrossRef]
- Wang, H.; Qian, X.; An, X. Tb-coordination polymer-anchored nanocellulose composite film for selective and sensitive detection of ciprofloxacin. Carbohydr. Polym. 2022, 287, 119337. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhu, X.; Liu, J.; Zou, L.; Li, G.; Ye, B. Ratiometric fluorescence detection of ciprofloxacin using the terbium-based coordination polymers. Spectrochim. Acta Part A Mol. Biomol. 2022, 269, 120775. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Ahn, K.H. Detection of Ciprofloxacin in Urine through Sensitized Lanthanide Luminescence. Sensors 2016, 16, 2065. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.T.; Nagarajan, D.; Kaliyamoorthy, S.; Rathinam, B. Citrate Functionalized Zirconium-Based Metal Organic Framework for the Fluorescent Detection of Ciprofloxacin in Aqueous Media. Micromachines 2022, 13, 2097. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Surya, S.G.; Beduk, T.; Vijjapu, M.T.; Lamaoui, A.; Durmus, C.; Timur, S.; Shekhah, O.; Mani, V.; Amine, A.; et al. Metal-Organic Frameworks Meet Molecularly Imprinted Polymers: Insights and Prospects for Sensor Applications. ACS Appl. Mater. Interfaces Spectrosc. 2022, 14, 49399–49424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Li, W.; Wei, Y.; Wei, X.; Qu, F.; Zhang, Y.; Nie, P.; Feng, X.; He, Y. A novel ratiometric fluorescent probe for sensitive detection of jasmonic acid in crops. Anal. Chim. Acta 2023, 1244, 340844. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Yang, W.; He, X.; Zhao, S.; Zhang, X.; Tang, W.; Wang, J.; Yue, T.; Li, Z. Amino-Functionalized Al-MOF for Fluorescent Detection of Tetracyclines in Milk. J. Agric. Food Chem. 2019, 67, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dramou, P.; Song, Z.; Zheng, L.; Zhang, X.; Ni, X.; He, H. Lanthanide metal doped organic gel as ratiometric fluorescence probe for selective monitoring of ciprofloxacin. Microchem. J. 2022, 179, 107476. [Google Scholar] [CrossRef]
- Zhou, T.; Halder, A.; Sun, Y. Fluorescent Nanosensor Based on Molecularly Imprinted Polymers Coated on Graphene Quantum Dots for Fast Detection of Antibiotics. Biosensors 2018, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, M.; Getachew, N.; Díaz, K.; Díaz-García, M.; Chebude, Y.; Díaz, I. Synthesis of metal–organic frameworks in water at room temperature: Salts as linker sources. Green Chem. 2015, 17, 1500–1509. [Google Scholar] [CrossRef]
- Ge, J.; Liu, L.; Qiu, L.; Jiang, X.; Shen, Y. Facile synthesis of amine-functionalized MIL-53(Al) by ultrasound microwave method and application for CO2 capture. J. Porous Mater. 2016, 23, 857–865. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).