Simple Chromatographic Sensor with UV LED Optical Detection for Monitoring Patients Treated with Continuous Ambulatory Peritoneal Dialysis †
Abstract
1. Introduction
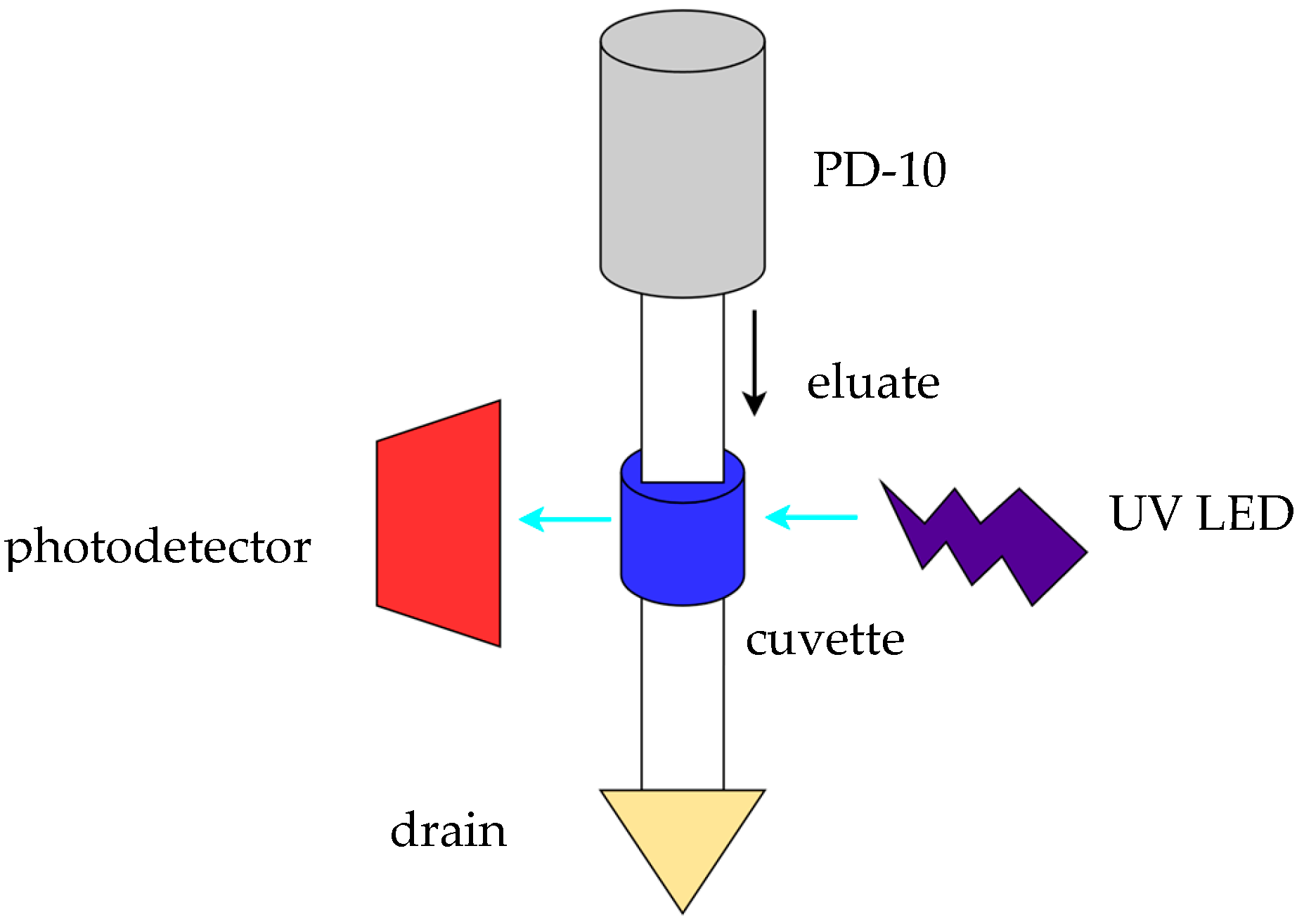
2. Materials and Methods
3. Results
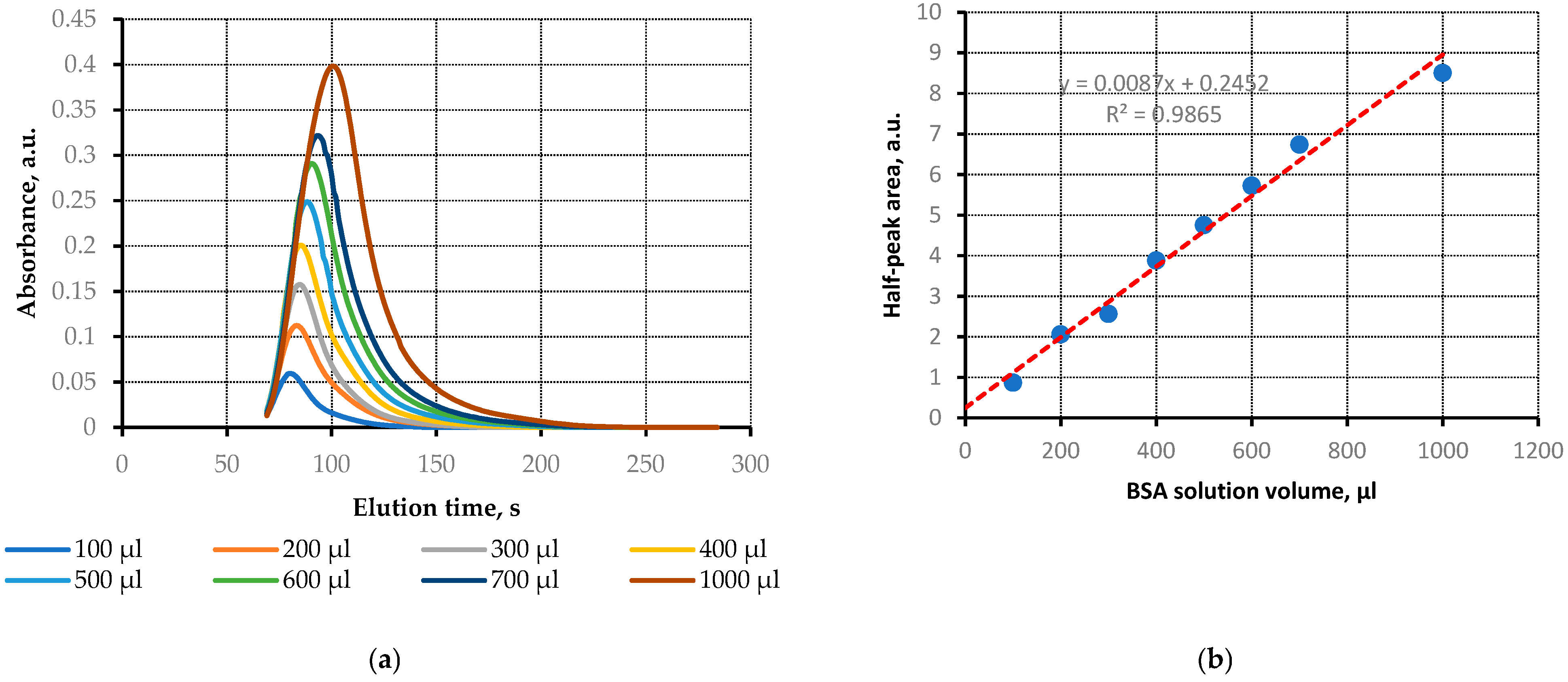
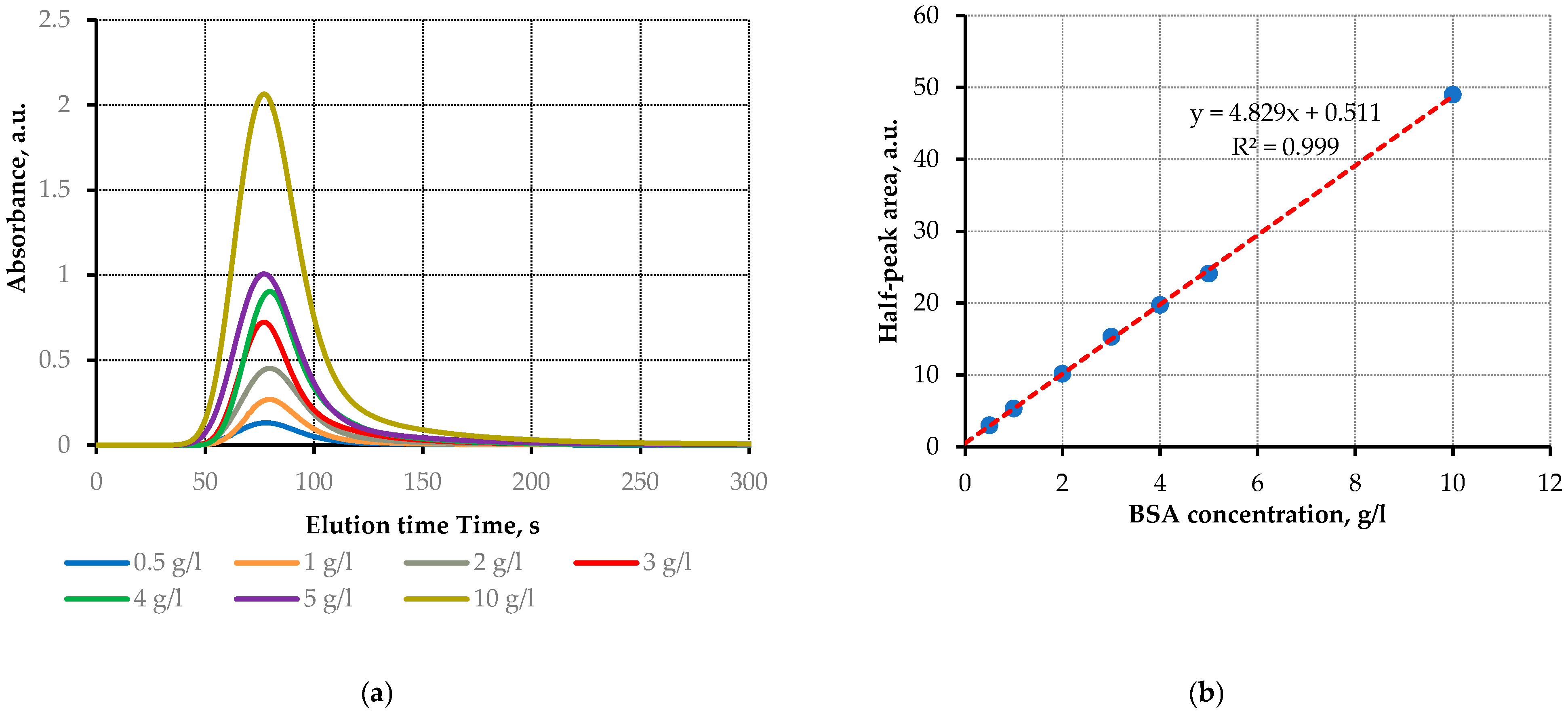
3.1. Sensor Calibration with BSA Solutions
3.2. Sensor Calibration with Nucleotides Solutions
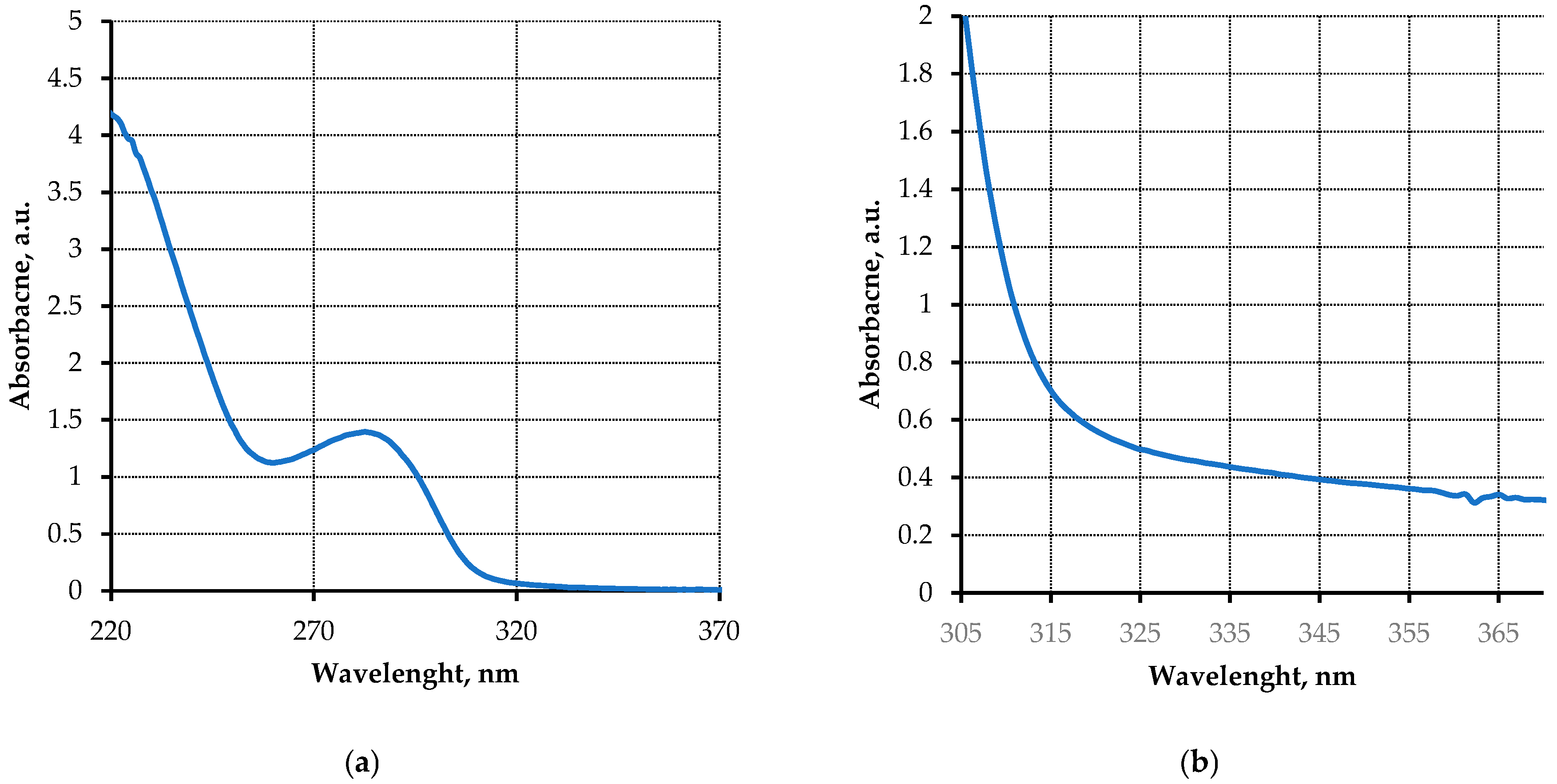
3.3. Chromatograms and UV Absorption Spectra of Effluent Peritoneal Dialysate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.A.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Chow, K.M.; Van de Luijtgaarden, M.W.M.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2017, 13, 90–103. [Google Scholar] [CrossRef]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The Current State of Peritoneal Dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, M.J.; Gahl, G.M.; Kopple, J.D.; Kamdar, A.V.; Jones, M.R.; Kessel, M.; Coburn, J.W. Protein losses during peritoneal dialysis. Kidney Int. 1981, 19, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ye, H.; Zhang, X.; Cao, P.; Guo, Q.; Mao, H.; Yu, X.; Yang, X. Association of Lean Body Mass Index and Peritoneal Protein Clearance in Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2019, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.I.; Kang, E.W.; Lee, Y.K.; Shin, S.K. Higher Peritoneal Protein Clearance as a Risk Factor for Cardiovascular Disease in Peritoneal Dialysis Patient. PLoS ONE 2013, 8, e56223. [Google Scholar] [CrossRef]
- Lu, W.; Pang, W.-F.; Jin, L.; Li, H.; Chow, K.M.; Kwan, B.C.-H.; Leung, C.B.; Li, P.K.-T.; Szeto, C.C. Peritoneal protein clearance predicts mortality in peritoneal dialysis patients. Clin. Exp. Nephrol. 2018, 23, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, Y.; Luo, S.; Xu, R.; Xu, Y. Peritoneal Protein Leakage, Systemic Inflammation, and Peritonitis Risk in Patients on Peritoneal Dialysis. Perit. Dial. Int. 2013, 33, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Fung, W.W.-S.; Li, P.K.-T. Recent advances in novel diagnostic testing for peritoneal dialysis-related peritonitis. Kidney Res. Clin. Pract. 2022, 41, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Lingervelder, D.; Koffijberg, H.; Kusters, R.; IJzerman, M.J. Point-of-care testing in primary care: A systematic review on implementation aspects addressed in test evaluations. Int. J. Clin. Pract. 2019, 73, e13392. [Google Scholar] [CrossRef] [PubMed]
- Walls, D.; Loughran, S.T. Protein Chromatography: Methods and Protocols, 2011st ed.; Humana Press: Totowa, NJ, USA, 2011; p. 681. ISBN 9781607619123. [Google Scholar]
- Determann, H. Gel Chromatography, Gel Filtration, Gel Permeation, Molecular Sieves: A Laboratory Hand Book, 2nd ed.; Springer: New York, NY, USA, 1969; p. 208. ISBN 978-3642950841. [Google Scholar]
- Sünter, A.; Frorip, A.; Korsakov, V.; Kurruk, R.; Kuznetsov, A.; Ots-Rosenberg, M. Optical method for screening and a new proteinuria focus group. J. Biomed. Photonics Eng. 2015, 1, 236–247. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Frorip, A.; Sünter, A.; Kasvand, N.; Korsakov, V.; Konoplev, G.; Stepanova, O.; Rusalepp, L.; Anton, D.; Püssa, T.; et al. Fast Protein and Metabolites (Nucleotides and Nucleosides) Liquid Chromatography Technique and Chemical Sensor for the Assessment of Fish and Meat Freshness. Chemosensors 2023, 11, 69. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Frorip, A.; Sünter, A.; Korsakov, V.; Konoplev, G.; Stepanova, O.; Roschina, N.; Ovsyannikov, N.; Lialin, D.; Gerasimchuk, R.; et al. Optical Chemical Sensor Based on Fast-Protein Liquid Chromatography for Regular Peritoneal Protein Loss Assessment in End-Stage Renal Disease Patients on Continuous Ambulatory Peritoneal Dialysis. Chemosensors 2022, 10, 232. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Frorip, A.; Gerasimchuk, R.P.; Konoplev, G.A.; Rosenberg, M.; Sinijärv, H.; Sünter, A.; Vasilevsky, A.M.; Zemchenkov, A.Y. MP479 Multimode monitoring of age excretion in hemodialysis. Nephrol. Dial. Transplant. 2016, 31, i500–i501. [Google Scholar] [CrossRef]
- Stinghen, A.E.M.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Y.; Chen, J.; Yang, X.; Mei, C.; Xiong, F.; Shi, W.; Zhou, W.; Liu, X.; Sun, S.; et al. Serum and Tissue Levels of Advanced Glycation End Products and Risk of Mortality in Patients on Maintenance Hemodialysis. Am. J. Nephrol. 2021, 52, 8–16. [Google Scholar] [CrossRef] [PubMed]
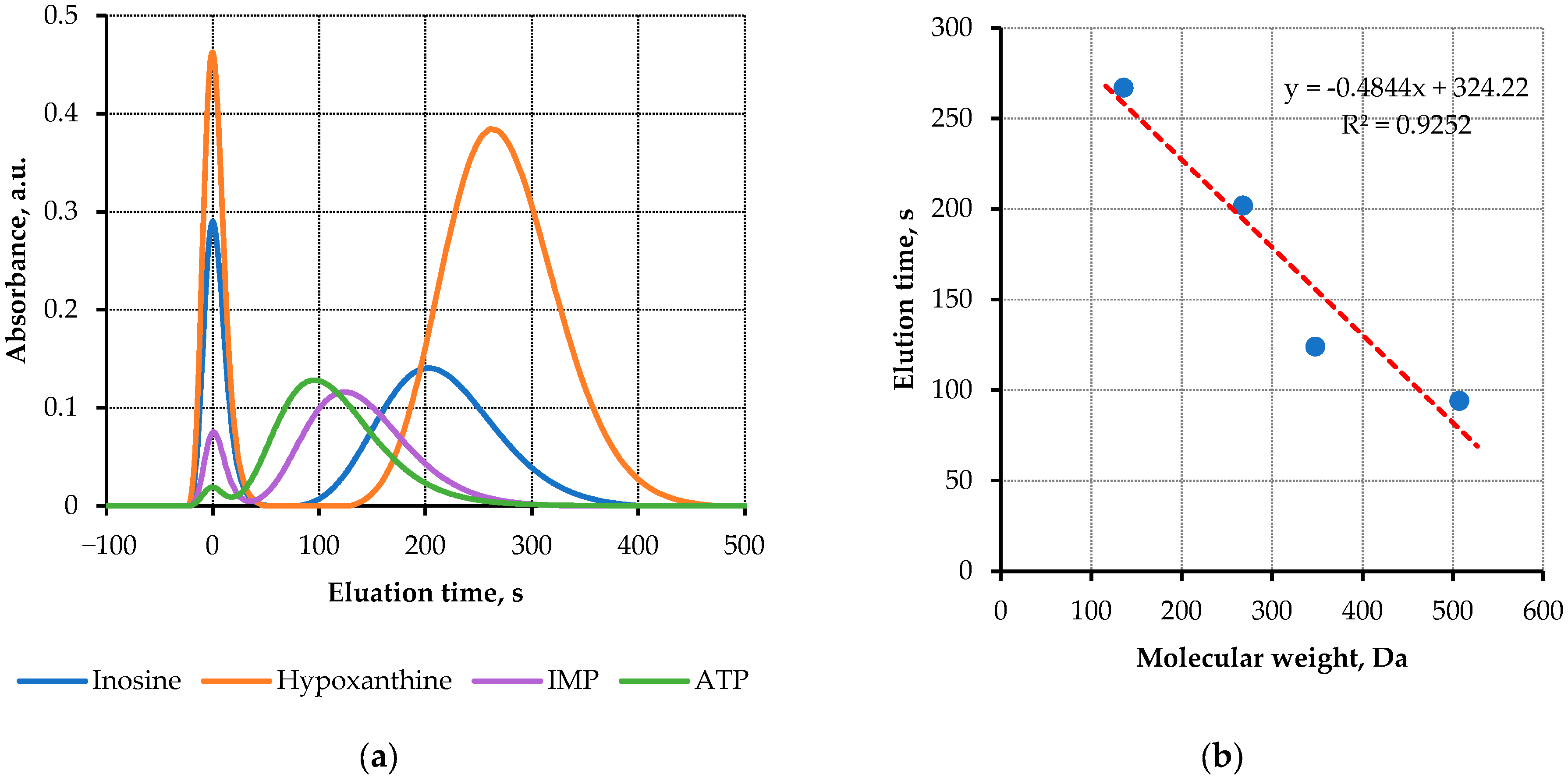

| Substance | Molecular Weight, Da | Elution Time, s |
|---|---|---|
| ATP | 507 | 94 |
| IMP | 348 | 124 |
| Ino | 268 | 202 |
| Hx | 136 | 267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konoplev, G.; Kuznetsov, A.; Frorip, A.; Sünter, A.; Korsakov, V.; Stepanova, O.; Roschina, N.; Ovsyannikov, N.; Gerasimchuk, R.; Isachkina, A.; et al. Simple Chromatographic Sensor with UV LED Optical Detection for Monitoring Patients Treated with Continuous Ambulatory Peritoneal Dialysis. Eng. Proc. 2023, 35, 25. https://doi.org/10.3390/IECB2023-14595
Konoplev G, Kuznetsov A, Frorip A, Sünter A, Korsakov V, Stepanova O, Roschina N, Ovsyannikov N, Gerasimchuk R, Isachkina A, et al. Simple Chromatographic Sensor with UV LED Optical Detection for Monitoring Patients Treated with Continuous Ambulatory Peritoneal Dialysis. Engineering Proceedings. 2023; 35(1):25. https://doi.org/10.3390/IECB2023-14595
Chicago/Turabian StyleKonoplev, Georgii, Artur Kuznetsov, Aleksandr Frorip, Alar Sünter, Vadim Korsakov, Oksana Stepanova, Natalia Roschina, Nikolay Ovsyannikov, Roman Gerasimchuk, Alina Isachkina, and et al. 2023. "Simple Chromatographic Sensor with UV LED Optical Detection for Monitoring Patients Treated with Continuous Ambulatory Peritoneal Dialysis" Engineering Proceedings 35, no. 1: 25. https://doi.org/10.3390/IECB2023-14595
APA StyleKonoplev, G., Kuznetsov, A., Frorip, A., Sünter, A., Korsakov, V., Stepanova, O., Roschina, N., Ovsyannikov, N., Gerasimchuk, R., Isachkina, A., Rustamova, Z., & Pavshukova, A. (2023). Simple Chromatographic Sensor with UV LED Optical Detection for Monitoring Patients Treated with Continuous Ambulatory Peritoneal Dialysis. Engineering Proceedings, 35(1), 25. https://doi.org/10.3390/IECB2023-14595






