Abstract
In this study, a low-cost, miniaturized fluorescence-based measurement system for optical biosensors has been developed. A 3D-printed setup with a blue light-emitting diode (LED) and photodiode was used for electrical detection and monitoring of fluorescence light intensity. The system was used to explore the fluorescence quenching of boron-dipyrromethene (Bodipy) in the presence of boronic acid functionalized benzyl viologen (o-BBV) to develop a monosaccharide detection platform by studying at different pHs and temperatures. The results showed that the system has potential for further development and optimization. This study provides a proof-of-concept for a low-cost and miniaturized optical biosensor for monosaccharides.
1. Introduction
Fluorescence is a technique that is widely acknowledged for its outstanding ability to detect with great sensitivity and specificity. Moreover, it is a non-invasive or minimally invasive method that facilitates non-destructive measurements and, occasionally, in vivo measurements [1]. Particularly, fluorescent-based techniques may apply to various scientific disciplines, including biochemistry, analytical chemistry, and sensor technologies [2]. In these techniques, fluorescence probes become the most effective tools that are sensitive to analytes, such as oxygen, pH, or specific ions, such as Ca2+, Zn2++, Na+, and K+ [3,4,5]. Among these various kinds of analytes, monosaccharides are one of the significant constituents in living organisms since they serve a critical function in several physiological processes, including metabolism and cellular recognition, thereby their detection becomes important [6].
The application of boronic acid, which forms complexes with cis-1,2- and 1,3-diols of sugar molecules, represents a widely used method for detecting monosaccharides [7]. The initial research in this area was conducted by Czernik and Shinkai, and subsequent reports have provided further evidence of the feasibility of utilizing boronic acid derivatives as a means of sensing sugar molecules [8]. In these reports, signal transduction is achieved through the reversible binding of a dye functionalized with boronic acid to monosaccharides, resulting in changes to the dye optical properties. This enables the detection and quantification of sugar concentrations in solution through fluorescence. However, this methodology relies on the integration of boronic acid to a fluorophore via covalent bonding, which limits the UV excitation of the dye and its photostability [9]. To overcome this limitation, an alternative approach has been devised, whereby the sensing system is separated into quencher and dye combinations that can serve as signal transducers for the detection and quantification of sugars [10]. Camara et al. developed the combination of trisodium-8-hydroxy-1,3,6-pyrenetrisulfonate (HPTS) as an anionic dye and ortho-boronic acid substituted benzyl viologen (o-BBV) as a quencher, determines the monosaccharides in the range of 0–1800 mg/dL [10]. Sharrett et al. synthesized multiple derivatives of bis-viologen-linked boronic acid as quenchers, while still utilizing HPTS as the fluorescent dye molecule, successfully detecting glucose concentrations within the 0–40 mM range [11]. Resendez et al. employed the identical sensing platform (HPTS/BBV) to explore the response towards fucose, L-rhamnose, and xylose [12]. As evidenced by the literature review, HPTS has been utilized as a fluorescent probe in a two-compartment detection system for the purpose of detecting monosaccharides [13,14]. Building upon this foundation, we elected to employ a Bodipy-based dye as a fluorescent probe. Despite possessing numerous advantageous optical properties, such as high fluorescence emission within the 510–800 nm range, a high absorption coefficient (ε > 50,000), high fluorescence quantum yield (ϕ > 70%), as well as high photostability and chemical stability [15], surprisingly this dye has not previously been employed in combination with the BBV quencher within a two-compartment sensing system designed for detecting monosaccharides.
The detection of monosaccharides has practical applications in food technology. The determination of the composition and levels of free sugars, including supplementary and natural sugars, in fruits, vegetables, and their processed products is significant in the food industry. For instance, glucose is found in grapes, oranges, apples, and bananas, as well as vegetables, such as sweet potatoes, carrots, and corn. Glucose is also a common ingredient in processed foods and is often added as a sweetener or to enhance the texture and flavor of foods, such as baked goods, energy bars, and sports drinks. Galactose is also present in smaller amounts in other foods, including some fruits and vegetables, such as apples, oranges, tomatoes, brussels sprouts, and broccoli. Galactose is used as a food additive in some processed foods and can be found in certain sweeteners, such as galactose syrup. Fructose is found in fruits such as apples, pears, bananas, berries, and melons. It is also found in high-fructose corn syrup, which is a common sweetener used in many processed foods, including soft drinks, baked goods, and snack foods. Fructose is also a component of honey and agave nectar, which are natural sweeteners used in cooking and baking [16]. Utilizing instrumental techniques is necessary for determining the concentration of simple sugars, particularly in processed foods. These instrumental techniques employed for quantifying the monosaccharides usually involve the use of sophisticated instruments, such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS), or capillary electrophoresis (CE) [17,18,19,20]. While these techniques offer exceptional sensitivity in quantification, they do come with a few drawbacks, such as requiring bulky and costly equipment, and being time-consuming.
Herein, we developed a fluorescence-based system using a custom-made 3D-printed setup for electrical detection and monitoring of light intensity of a Bodipy based fluorescent dye. The system utilizes a blue LED and a photodiode to optically excite the Bodipy dye in a solution and capture the resulting fluorescence light, generating a measurable electrical current. The system is applied to investigate the ratiometric fluorescence quenching of the fluorescent Bodipy in the presence of the quencher o-BBV, with the goal of the detection of glucose, galactose, and fructose as building blocks of the monosaccharides. This study aims to provide a low-cost, miniaturized alternative to bulky and expensive optical equipment for biosensing applications of the monosaccharides.
2. Materials and Methods
2.1. Fluorescent Measurement Setup
The custom-made 3D-printed setup (Figure 1) was constructed for monitoring the fluorescence light intensity electrically. This setup provided a low-cost and compact alternative to the large and expensive optical equipment and spectrometers generally used for measuring the absorption or emission of light intensity of a solution. In the experimental setup, a blue LED with a wavelength of 450 nm and a photodiode (PD) were positioned orthogonally on the top and backside of a cell package, respectively. The photodiode was operated under a reverse bias of 1V, thus yielding an electrical current correlated with the intensity of the fluorescent light of the solution. The LED was powered with a continuous DC bias of 30 mA and 2.63 V, operating at an approximate power output of 80 mW. This configuration allowed for minimizing the size of a glucose sensor, making it more accessible and cost-effective. The system was built in such a way that direct light from the LED did not reach the PD. Any exterior light was blocked after inserting a quartz cell into the cell package and sealing the cover. The blue LED then illuminated the fluorescent solution, the fluorescent light was captured by the PD and translated into an electric current.
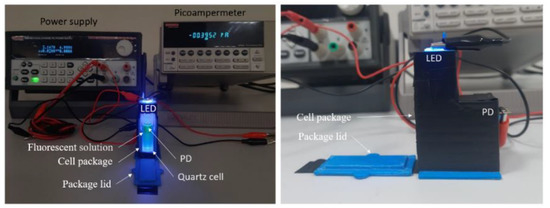
Figure 1.
Representation of the designed electrical-based fluorescent measurement setup, the front side (left image), and the right side of the device (right image).
2.2. Quenching Process
The quencher (o-BBV) and the fluorescent dye (a Bodipy derivative) have been synthesized in a previous study [21]. For determining the optimal quenching conditions, various concentrations of o-BBV (1 × 10–5, 1 × 10–4, 1 × 10–3, and 1 × 10–2 M) were added to a solution of Bodipy (2 × 10–6 M and 4 × 10–6 M, 3.4 mL) in a quartz cell. The mixture was stirred for 120 s while exposed to UV light during the quenching process. Following quenching, the fluorescence intensity of the solution was measured using the custom-made 3D-printed setup at various time intervals, including 30, 60, and 120 s. The resulting measurements were averaged and compared with the results collected from the unquenched solution.
2.3. Effect of the Temperature and pH on the Emission Intensity of the Bodipy
Bodipy solutions were prepared in ethanol:PBS (1:1) at different pH values (5.5, 7.4, and 8.0) and temperatures ranging from 0 °C to 60 °C. The fluorescence emission intensities of the Bodipy samples were measured using a blue LED (450 nm), and the resulting currents (nA) were recorded at 30, 60, and 120 s intervals. The average current values were obtained and used to assess the effect of pH and temperature on the Bodipy fluorescence response.
2.4. Glucose, Fructose and Galactose Responses
The fluorescence response of a quenched Bodipy solution to increasing concentrations of glucose was investigated in this study. 200 μL of glucose solutions ranging from 10 mM to 100 mM were added to the 5 mL of quenched Bodipy solution and the resulting fluorescence intensity was measured at a pH: 7.4 and a temperature of 25 °C. Additionally, fructose and galactose response were studied by following the same procedure.
3. Results and Discussion
3.1. The Study of the Quenching Efficiency on Bodipy Concentrations
Two different concentrations of Bodipy solution (2 × 10–6 M and 4 × 10–6 M) were subjected to treatment with varying concentrations of o-BBV at pH 7.4, in order to observe their quenching behaviors (Figure 2). The fluorescence intensity of the low concentration of Bodipy resulted in 20 nA in the PD system, while the high concentration of Bodipy yielded 60 nA. The low concentrations of o-BBV (1 × 10–4 M and 1 × 10–5 M) did not significantly alter the emission intensity of Bodipy. However, high concentration of o-BBV (1 × 10–2 M) led to a reduction in the illumination for both concentrations of Bodipy (2 × 10–6 M and 4 × 10–6 M) by 30% and 33%, respectively, after 10 min of UV light exposure.
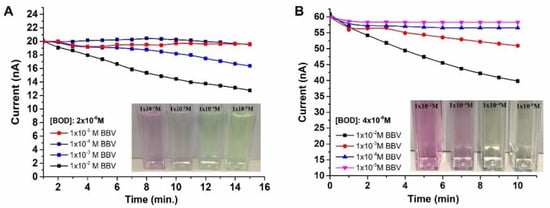
Figure 2.
Photodiode current versus time under LED excitation of Bodipy solutions with concentrations of 2 × 10–6 M (A) and 4 × 10–6 M (B).
The impact of stirring on the interaction between Bodipy and o-BBV was examined using the most effective quenched dual system, consisting of concentrations of 4 × 10−6 M and 1 × 10−2 M for Bodipy and o-BBV, respectively (Figure 3A). Stirring the mixture of Bodipy and o-BBV mixture at 400 rpm for 5 min resulted in a 5% enhancement in quenching efficiency. Moreover, the temperature effect on the emission intensity of Bodipy in ethanol: PBS at pH 5.5, 7.4, and 8.0 was also studied at 0, 4, 25, 37, and 60 °C (Figure 3B). The results demonstrated that Bodipy exhibited high emission intensity below and above room temperatures for both mildly acidic (pH: 5.5) and basic conditions (pH: 8.0). For pH:7.4, the emission intensity of Bodipy remained stable at 25, 37, and 60 °C.
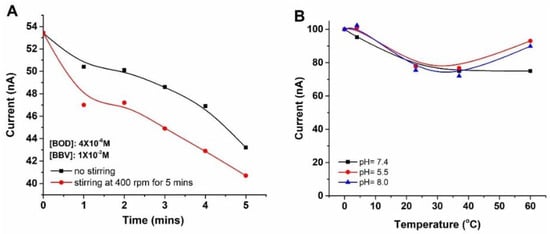
Figure 3.
Stirring effect on the quenching efficiency (A), and temperature effect on the emission intensity of Bodipy at different pH values (B).
3.2. Investigation the Quenching Behavior at Different pH and Temperatures
Figure 2 clearly indicates that at high concentrations of Bodipy and o-BBV solutions, a pink color was observed in the final solution, suggesting the formation of a complexation between the molecules. The mechanism of this complexation has been previously reported in detail in the literature. To achieve a transparent final mixture solution, a concentration range of 1 × 10–2 M to 1 × 10–3 M of o-BBV was chosen for the quenching experiments, as the aim was to restore the emission intensity of Bodipy upon addition of glucose. Therefore, 2 × 10–6 M of Bodipy solution was mixed with 2 × 10–3 M of o-BBV at pH 5.5, 7.4 and 8.0 at 0, 4, 25, 37 and 60 °C to yield different quenching behaviors (Figure 4). The quenching efficiency of o-BBV on the fluorescent dye was investigated in this study at various pH and temperature values. The results showed successful quenching of the fluorescent dye by o-BBV at all pH and temperature values, except at pH 7.4 and 60 °C, where no reduction in the emission intensity of the Bodipy was observed (Figure 4B). The best quenching efficiency was observed at pH 7.4 for low temperatures (0 and 4 °C) and pH 8.0 for high temperatures (40 and 60 °C) (Figure 4B,C). The difference in quenching efficiency at different pH and temperature values could be attributed to the molecular dynamics of both the quencher and fluorescent molecule.
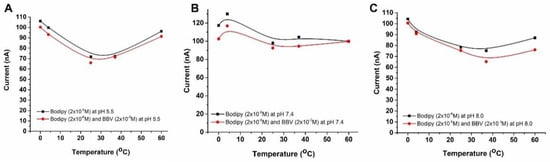
Figure 4.
Photodiode currents versus temperature under LED excitation of Bodipy solutions before and after the addition of BBV under different pH levels of 5.5 (A), 7.4 (B), and 8.0 (C)
3.3. Study of Glucose, Fructose and Galactose Sensing
A custom-made 3D-printed sensing platform was utilized to determine the sensing capability of glucose, fructose, and galactose. The recovery of Bodipy emission was observed in all monosaccharides at a pH of 7.4 and a temperature of 25 °C (Figure 5). Linearity was established between concentrations of 10–30 mM, 10–70 mM, and 10–60 mM for glucose, fructose, and galactose, respectively (Figure 5). These findings provide valuable insights into the potential use of this setup for detecting and quantifying various monosaccharides in biological and environmental samples.
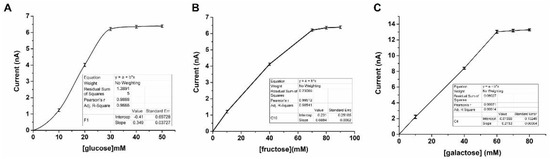
Figure 5.
Photodiode currents under LED excitation of Bodipy solutions showing the recovery of emission after the addition of glucose (A), fructose (B), and galactose (C) under various concentrations.
4. Conclusions
In summary, our study found that o-BBV is capable of effectively quenching the fluorescence emission of Bodipy at mild acidic, neutral, and mild basic conditions while the temperatures were at 0 °C, 4 °C, 25 °C, 37 °C, and 60 °C. Our custom-made 3D-printed optical biosensor was successful in detecting low concentrations of glucose, fructose, and galactose in the range of 10–70 mM. The simplicity, low cost, and portability of the biosensor make it a promising alternative to conventional fluorescence measurement methods. Additionally, the detection of monosaccharides in foods by this device is a significant breakthrough in the field of food safety and nutrition. With the help of this device, it will be possible to detect the presence of various monosaccharides in food products, which can help to determine their level in a specific range. Future research could focus on improving the biosensor’s sensitivity and selectivity by exploring the use of different dyes and quenchers to enhance the sensor’s detection range and specificity. Moreover, investigating the biosensor’s stability under various conditions is crucial to determine its resilience for real-world applications. Shrinking the size of biosensor could potentially lead to the development of a portable and monitoring the monosaccharide levels in food.
Author Contributions
O.K. conducted experiments, analyzed data, and contribute the writing, M.İ.B. designed, built, and optimized the optical based sensor system and reviewed the manuscript, and S.D.T. determined the concept of the study, designed experiments, analyzed data, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be provided upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Basabe-Desmonts, L.; Reinhoudt, D.N.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Drummen, G.P.C. Fluorescent probes and fluorescence (microscopy) techniques-illuminating biological and biomedical research. Molecules 2012, 17, 14067–14090. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.G.; Yuan, Q.; Lv, P.; Chen, K. Research progress of small molecule fluorescent probes for detecting hypochlorite. Sensors 2021, 21, 6326. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Xu, S.; Li, C.; Dong, B. Recent Progress in Fluorescent Probes For Metal Ion Detection. Front. Chem. 2022, 10, 5241. [Google Scholar] [CrossRef] [PubMed]
- Sosicka, P.; Ng, B.G.; Freeze, H.H. Therapeutic Monosaccharides: Looking Back, Moving Forward. Biochemistry 2020, 59, 3064–3077. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Liu, A.; Li, Y.; Fang, G.; Yao, Q.; Zhang, G.; Wu, Z. Boronic acid sensors with double recognition sites: A review. Analyst 2020, 145, 719–744. [Google Scholar] [CrossRef]
- Kawanishi, T.; Romey, M.A.; Zhu, P.C.; Holody, M.Z.; Shinkai, S. A study of boronic acid based fluorescent glucose sensors. J. Fluoresc. 2004, 14, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Pappin, B.; Kiefel, M.J.; Houston, T.A. Houston, Boron-Carbohydrate Interactions. 2012. Available online: https://books.google.com.hk/books?hl=zh-TW&lr=&id=V9mgDwAAQBAJ&oi=fnd&pg=PA37&dq=Houston,+Boron-Carbohydrate+Interactions&ots=rcj28pWXFu&sig=OV6MS2Vul7I1ABAU29_VLb74NS8&redir_esc=y#v=onepage&q=Houston%2C%20Boron-Carbohydrate%20Interactions&f=false (accessed on 8 May 2023).
- Camara, J.N.; Suri, J.T.; Cappuccio, F.E.; Wessling, R.A.; Singaram, B. Boronic acid substituted viologen based optical sugar sensors: Modulated quenching with viologen as a method for monosaccharide detection. Tetrahedron Lett. 2002, 43, 1139–1141. [Google Scholar] [CrossRef]
- Sharrett, Z.; Gamsey, S.; Levine, P.; Cunningham-Bryant, D.; Vilozny, B.; Schiller, A.; Wessling, R.A.; Singaram, B. Boronic acid-appended bis-viologens as a new family of viologen quenchers for glucose sensing. Tetrahedron Lett. 2008, 49, 300–304. [Google Scholar] [CrossRef]
- Resendez, A.; Halim, M.A.; Singh, J.; Webb, D.L.; Singaram, B. Boronic acid recognition of non-interacting carbohydrates for biomedical applications: Increasing fluorescence signals of minimally interacting aldoses and sucralose. Org. Biomol. Chem. 2017, 15, 9727–9733. [Google Scholar] [CrossRef] [PubMed]
- António, J.P.M.; Russo, R.; Carvalho, C.P.; Cal, P.M.S.D.; Gois, P.M.P. Boronic acids as building blocks for the construction of therapeutically useful bioconjugates. Chem. Soc. Rev. 2019, 48, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- Resendez, A.; Panescu, P.; Zuniga, R.; Banda, I.; Joseph, J.; Webb, D.L.; Singaram, B. Multiwell Assay for the Analysis of Sugar Gut Permeability Markers: Discrimination of Sugar Alcohols with a Fluorescent Probe Array Based on Boronic Acid Appended Viologens. Anal. Chem. 2016, 88, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tester, R.F. Fructose, galactose and glucose—In health and disease. Clin. Nutr. ESPEN 2019, 33, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Khan, N.M.; Nunez, K.M.; Chess, E.K.; Szabo, C.M. Complete monosaccharide analysis by high-performance anion-exchange chromatography with pulsed amperometric detection. Anal. Chem. 2012, 84, 4104–4110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; She, L.; Xu, Z.Y.; Wang, Z.K.; Ma, Z.; Yang, F.; Li, Z.T. A BODIPY-modified polymeric micelle for sustaining enhanced photodynamic therapy. Chin. Chem. Lett. 2022, 33, 3277–3280. [Google Scholar] [CrossRef]
- Daikuzono, C.M.; Delaney, C.; Tesfay, H.; Florea, L.; Oliveira, O.N.; Morrin, A.; Diamond, D. Impedance spectroscopy for monosaccharides detection using responsive hydrogel modified paper-based electrodes. Analyst 2017, 142, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Glyad, V.M. Determination of monosaccharides, disaccharides, and oligosaccharides in the same plant sample by high-performance liquid chromatography. Russ. J. Plant Physiol. 2002, 49, 277–282. [Google Scholar] [CrossRef]
- Demirel Topel, S.; Beyaz, M.İ. Fluorescence quenching-based bodipy-boronic acid linked viologen dual system for potential glucose sensing applications. Sens. Rev. 2022, 42, 62–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).