1. Introduction
The human body is composed of different biomolecules. Broadly, they are classified into four large groups, carbohydrates, fats, proteins, and nucleic acids, with minor concentrations of other signaling molecules such as hormones, etc. Each of the biomolecules in turn comprise of four key elements—Oxygen, Carbon, Hydrogen, and Nitrogen. The composition of biomolecules that make up different tissues varies both between the tissue types spatially and varies within the same tissue temporally. This spatial and temporal variation is essential for healthy functioning of the human body. For example, blood has a different composition to muscles which is different from bones and is spatially segregated. Even within a tissue, there are spatial variances at different scales. A good example of such spatial variation in composition is a skeletal muscle that is attached to bones via tendons. The muscle itself is made of sarcomeres and myofibrils which differ in their molecular composition. Tissues develop variations in their biochemical composition over time like the healing of an open wound where a clot develops by the development of fibrin. White blood cells and macrophages clear the damaged tissue and foreign bodies. Over the healing process, new capillaries grow with new fibrous tissue development and skin recovery. The site of the biological process is not altered, but the composition changes temporally. During a diseased state, the composition of some of the biomolecules departs from the levels seen in healthy physiology. This shift from normal is the basis of clinical diagnoses such as blood tests, imaging techniques, etc. Clinical diagnostics are frequently invasive, cumbersome, and non-continuous. There is a need for repeated, reliable, non-invasive assessments of diseases that detects changes spatially like cancers and tumors, or temporally such as diabetes, sepsis, etc.
BioPhotonics diagnostic tools are capable of both spatial and temporal assessment of tissue, thus delivering the unmet need of repeated, reliable, non-invasive assessment in clinics. Light–tissue interaction is the basis of realizing diagnostic tools using BioPhotonics that are clinically relevant. When the biomolecules interact with light, the light is – absorbed, reflected, transmitted through, scattered—both linearly and non-linearly or transformed into a different colour through fluorescence by the biomolecule. Further, there can be heating or vibration-producing acoustic effects in the biomolecules [
1]. Each of these light-tissue interactions is utilized in BioPhotonics to develop an effective diagnostic tool tailored to the clinical problem at hand. In this paper, we will focus on optical spectroscopy, where a unique optical spectrum of the biomolecule of interest is extracted using light. This optical spectrum is then used to identify disease states. To understand the generation of the optical spectrum, we note that different wavelengths of light interact with the biomolecules differently, initiating either electronic transitions or exciting rotational or vibrational modes of the chemical bonds. Since each of the biomolecules is chemically distinct, they generate unique optical spectra. For obtaining the optical spectrum of the biomolecule of interest a suitable optical source is required. To select the appropriate source one has to be mindful of the safety limits in terms of the optical power. Also, one has to note that light of certain wavelengths, such as X-rays and far UV are harmful to the tissue due to their ability to ionize.
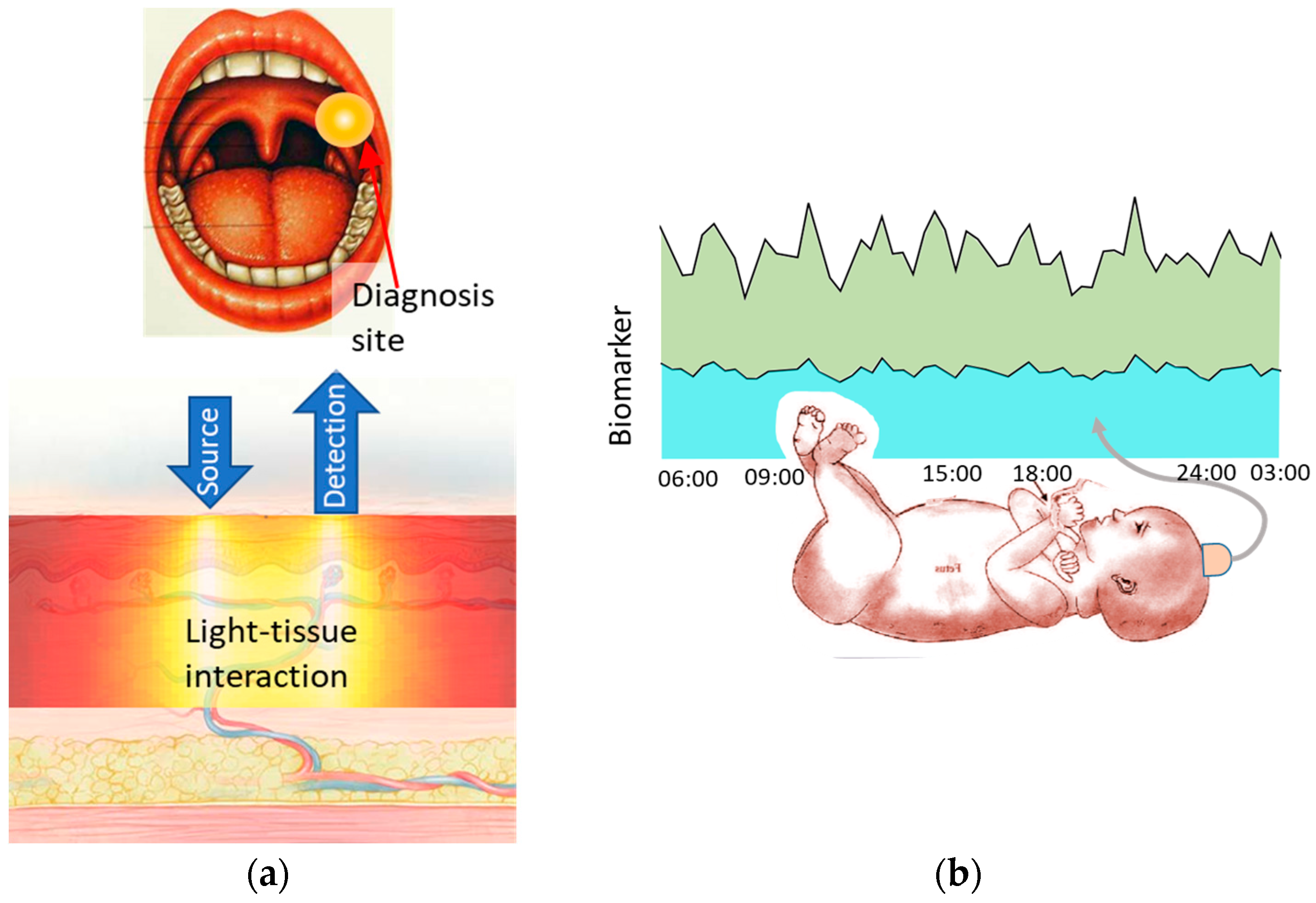
In this paper, we look at the role of optical spectroscopy in two scenarios as shown in
Figure 1. First in oral cancer-where spatial classification of tissue health is critical. Secondly in fetal hypoxia assessment-where a temporal evolution of biomolecules is monitored. Both these cases are based on the projects in the Author’s group (Biophotonics@Tyndall) where the Author is involved.
2. Spatial Assessment—Oral Cancer
Oral squamous cell carcinomas (OSCC) manifest in the early stages as painless white or red plaques in the mouth. Dysplastic cells under the oral skin accumulate many genetic alterations and mutations. This site now attracts macrophages while the immune response is suppressed. This site evolves into a high-grade dysplasia and then into invasive caner [
5]. Metabolism in this site is different to the healthy tissue in other parts of the oral cavity. The detection of such sites by local swab sampling followed by histopathology is the current diagnostic gold standard. However, swab samples only access the superficial cell layers.
Optical spectroscopy can be designed to penetrate the tissue to a depth, non-invasively, where dysplasia might exist. Further, a multimodal approach that involves diffused reflectance spectroscopy (DRS), Raman spectroscopy, and fluorescence spectroscopy is used to target biomolecules accurately that are effective in the recognition of cancerous sites spatially [
6].
3. Temporal Assessment—Fetal Hypoxia Assessment
Lack of oxygen, or hypoxia, in the baby during labour could result in death. The babies that survive this ordeal could be faced with developmental problems in their brain like cerebral palsy. Assessment of a hypoxic state is a critical clinical diagnostic event that allows the obstetrician to decide in favour of a Cesarean section (C-section). This time-critical diagnosis is currently done by monitoring fetal heart rate (FHR), which is an overall fetal well-being parameter, through fetal scalp electrodes or cardiotocographs (CTG). Oxygen content in the blood, SpO2, is a better correlate of hypoxia in the fetus and it is measured through fetal pulse oximetry. The current gold standard of hypoxia diagnosis is fetal blood sampling (FBS) where lactate and pH are measured in the fetal blood obtained from the fetal scalp using a scalpel. However, this method is invasive and can only be performed sporadically [
7]. Thus, there is an unmet need for a non-invasive, continuous diagnosis of hypoxia where the changes in hypoxia related biomolecules, namely lactate concentration and pH, concentrations are monitored in real-time during labour. This will aid in the assessment of onset of hypoxia in babies.
For spectroscopic assessment of hypoxia, lactate and pH levels need to be determined. Light in the long wavelength near-infrared (LW-NIR) spectrum, i.e., 1350–2500 nm, is a well-suited spectrum for the detection of these biomolecules. LWNIR region consists of combination and secondary absorption bands of small molecules like lactate, water, glucose and lipids. Water forms 70–90% of all our tissues. Lactate spectral features are superimposed on large water absorption regions, so the detection of lactate demands high sensitivity in detection in this region. The spectral features are broad and have many interferences for other molecules like glucose etc. Thus, a good spectroscopic tool is required to assess hypoxia in this region [
8,
9].
4. Conclusions and Future Outlook
In this paper, a perspective of BioPhotonics is based on clinically relevant, non-invasive diagnostic tools. Specifically, diagnostic tools with the ability to find malignant lesions in Oral cancer diagnosis earlier and with a higher accuracy was discussed that focused on spatial detection. Next, a tool to diagnose fetal distress during labour was discussed, including the challenges of building such a BioPhotonics tool. When implanted, this would result in a safer delivery both for the mother and the infant. Both these cases have a transformative impact on clinical diagnostic practice.
Looking ahead, BioPhotonics-based diagnostic tools provide a non-invasive, sustainable, reliable way to assess diseases as they evolve over both space and time. They will provide non-destructive, real-time tissue diagnosis for both daily life as wearables and as tools during surgeries.
Funding
This research was funded by the SFI-15/RP/2828 SFI Professorship Award and EI-CF20211693B EI commercialization fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created for this paper.
Acknowledgments
The author thanks the Biophotonics group@Tyndall National Institute and Irish Photonics Integration Center, South Infirmary Victoria University Hospital, Cork University Hospital, ENTO Research Institute, University College Cork, Dental School and Hospital, University College Cork, Cork Univ. Maternity Hospital (Ireland) and The INFANT Research Ctr. for providing the facilities and support for this work. Specifically, the author thanks the contributors Siddra Maryam, Marcelo Saito Nogueira, Rekha Gautam, Sanathana Konugolu Venkata Sekar, Kiang Wei Kho, Huihui Lu, Richeal Ni Riordain (ENTO Research Institute, University College Cork, Cork University Dental School and Hospital), Linda Feeley (ENTO Research Institute, University College Cork, Cork University Hospital), Patrick Sheahan (South Infirmary Victoria University Hospital, Cork University Hospital), Urbashi Basu (National Center for Biological Sciences, India, Princeton University, USA), Francesca Di Croce (University of Pavia, Italy), Walter Messina, Cleitus Antony, Paul Townsend, Fergus P. McCarthy (Cork Univ. Maternity Hospital (Ireland), the INFANT Research Ctr., Univ. College Cork (Ireland), Ray Burke and Stefan Andersson-Engels.
Conflicts of Interest
The author declares no conflict of interest.
References
- Tuchin, V.V. Tissue optics and photonics: Light-tissue interaction. J. Biomed. Photonics Eng. 2015, 1, 98–134. [Google Scholar] [CrossRef]
- Tik, J. The Tonsils and Other Soft Tissue Structures of the Mouth. Doctor’s Office Poster. Available online: https://www.flickr.com/photos/jantik/54322517/ (accessed on 14 February 2023).
- Stankovic, K.M.; Tan, O.T.; Sadow, P.M. Case 36-2015: A 27-year-old woman with a lesion of the ear canal. N. Engl. J. Med. 2015, 373, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Fatema, N.; Acharya, Y.; Al Yaqoubi, H. Amniotic Band Syndrome: A Silent Knife In-Utero. Nepal Med. Coll. J. 2019, 21, 153–159. [Google Scholar] [CrossRef]
- Rangel, R.; Pickering, C.R.; Sikora, A.G.; Spiotto, M.T. Genetic changes driving immunosuppressive microenvironments in oral premalignancy. Front. Immunol 2022, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Maryam, S.; Nogueira, M.S.; Gautam, R.; Krishnamoorthy, S.; Venkata Sekar, S.K.; Kho, K.W.; Lu, H.; Ni Riordain, R.; Feeley, L.; Sheahan, P.; et al. Label-Free Optical Spectroscopy for Early Detection of Oral Cancer. Diagnostic 2022, 12, 2896. [Google Scholar] [CrossRef] [PubMed]
- Al Wattar, B.H.; Honess, E.; Bunnewell, S.; Welton, N.J.; Quenby, S.; Khan, K.S.; Zamora, J.; Thangaratinam, S. Effectiveness of intrapartum fetal surveillance to improve maternal and neonatal outcomes: A systematic review and network meta-analysis. CMAJ 2021, 193, E468–E477. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Burke, R.; Mc Carthy, F.; Andersson-Engels, S. Beyond oxygen in-vivo long-wavelength near infra-red spectroscopy for hypoxia assessment. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 20–24 June 2021. [Google Scholar]
- Krishnamoorthy, S.; Basu, U.; Kho, K.; Gautam, R.; Di Croce, F.; Messina, W.; Antony, C.; Townsend, P.; McCarthy, F.P.; Andersson-Engels, S.; et al. Non-invasive continuous hypoxia assessment in intra-partum fetus through long wavelength near infrared spectroscopy. In SPIE Proceedings, Proceedings of Photonic Instrumentation Engineering X, San Francisco, CA, USA, 30 January–1 February 2023; SPIE: Bellingham, WA, USA; Volume 12428, pp. 176–178.
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).