Study of a pH-Sensitive Hologram for Biosensing Applications †
Abstract
:1. Introduction
2. Materials and Methods
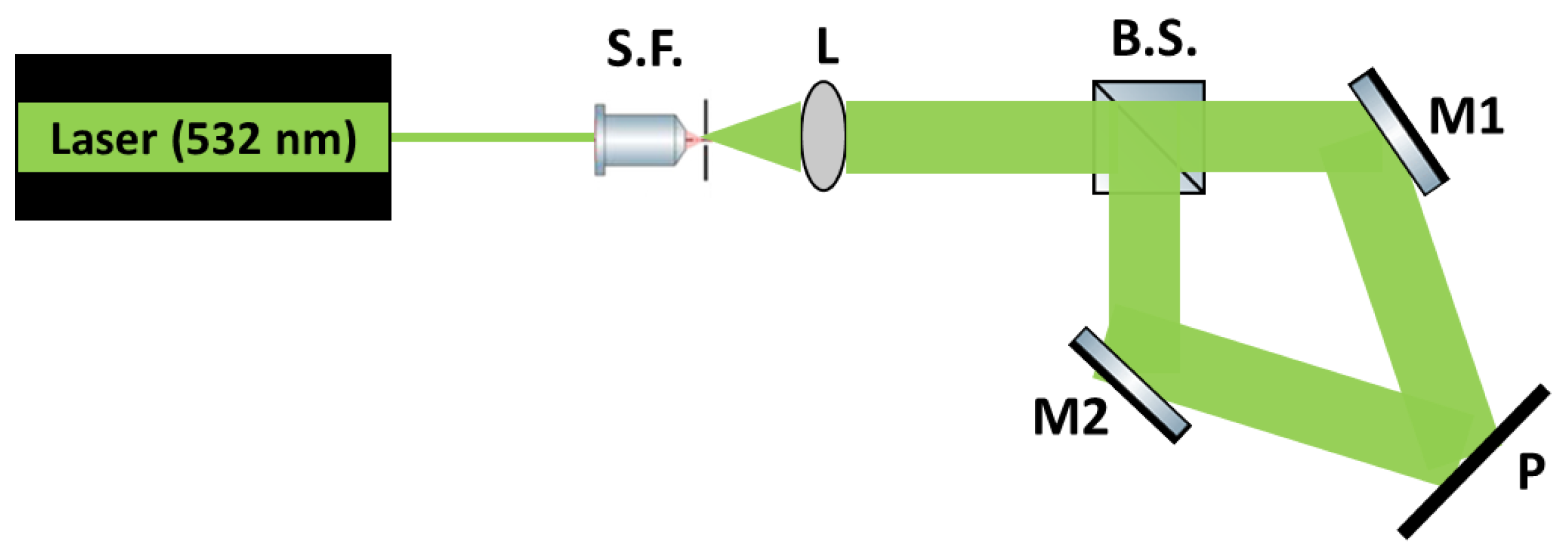
2.1. Recording Process
2.2. pH Sensitivity Measurement
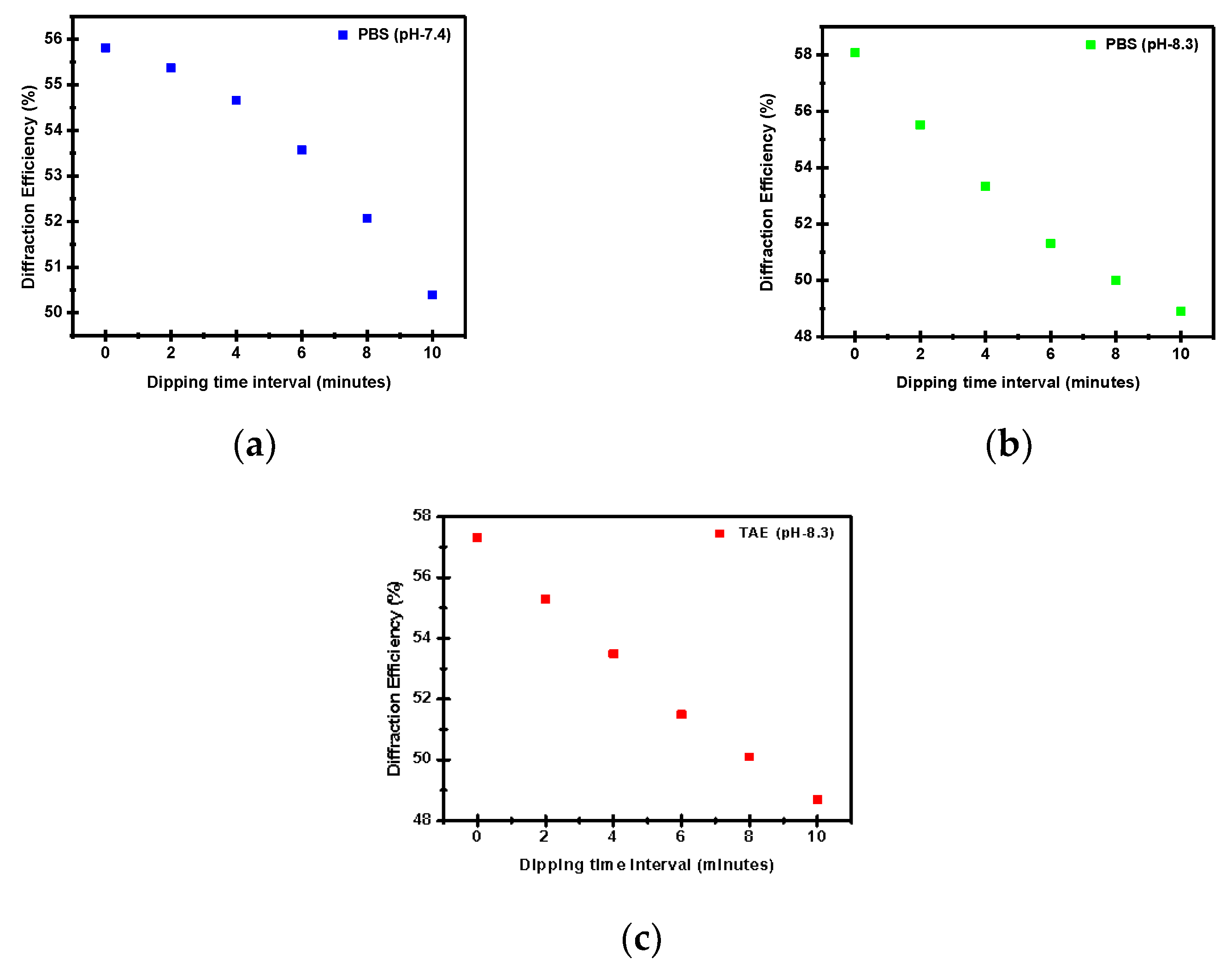
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Gai, Z.; Cheng, J.; Du, K.; Wei, W.; Li, Y.; Gao, Q.; Zou, C.; Qian, R.; Sun, Z.; et al. Construction of Beta-Cyclodextrin Modified Holographic Sensor for the Determination of Ibuprofen in Plasma and Urine. SSRN Electron. J. 2023, 385, 133650. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Akhtar, M.; Kadhum, A.; Mohamad, A.; Al-Amiery, A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Cassidy, J.; Naydenova, I. Water Resistant Cellulose Acetate Based Photopolymer for Recording of Volume Phase Holograms. Photonics 2021, 8, 329. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, K.; Heena; Mohanta, G.C.; Kumar, R. Study of a pH-Sensitive Hologram for Biosensing Applications. Eng. Proc. 2023, 34, 22. https://doi.org/10.3390/HMAM2-14272
Sharma K, Heena, Mohanta GC, Kumar R. Study of a pH-Sensitive Hologram for Biosensing Applications. Engineering Proceedings. 2023; 34(1):22. https://doi.org/10.3390/HMAM2-14272
Chicago/Turabian StyleSharma, Komal, Heena, Girish C. Mohanta, and Raj Kumar. 2023. "Study of a pH-Sensitive Hologram for Biosensing Applications" Engineering Proceedings 34, no. 1: 22. https://doi.org/10.3390/HMAM2-14272
APA StyleSharma, K., Heena, Mohanta, G. C., & Kumar, R. (2023). Study of a pH-Sensitive Hologram for Biosensing Applications. Engineering Proceedings, 34(1), 22. https://doi.org/10.3390/HMAM2-14272





