Comparative Analysis of Feature Extraction Methods for Intelligence Estimation Based on Resting State EEG Data †
Abstract
1. Introduction
2. Theoretical Background
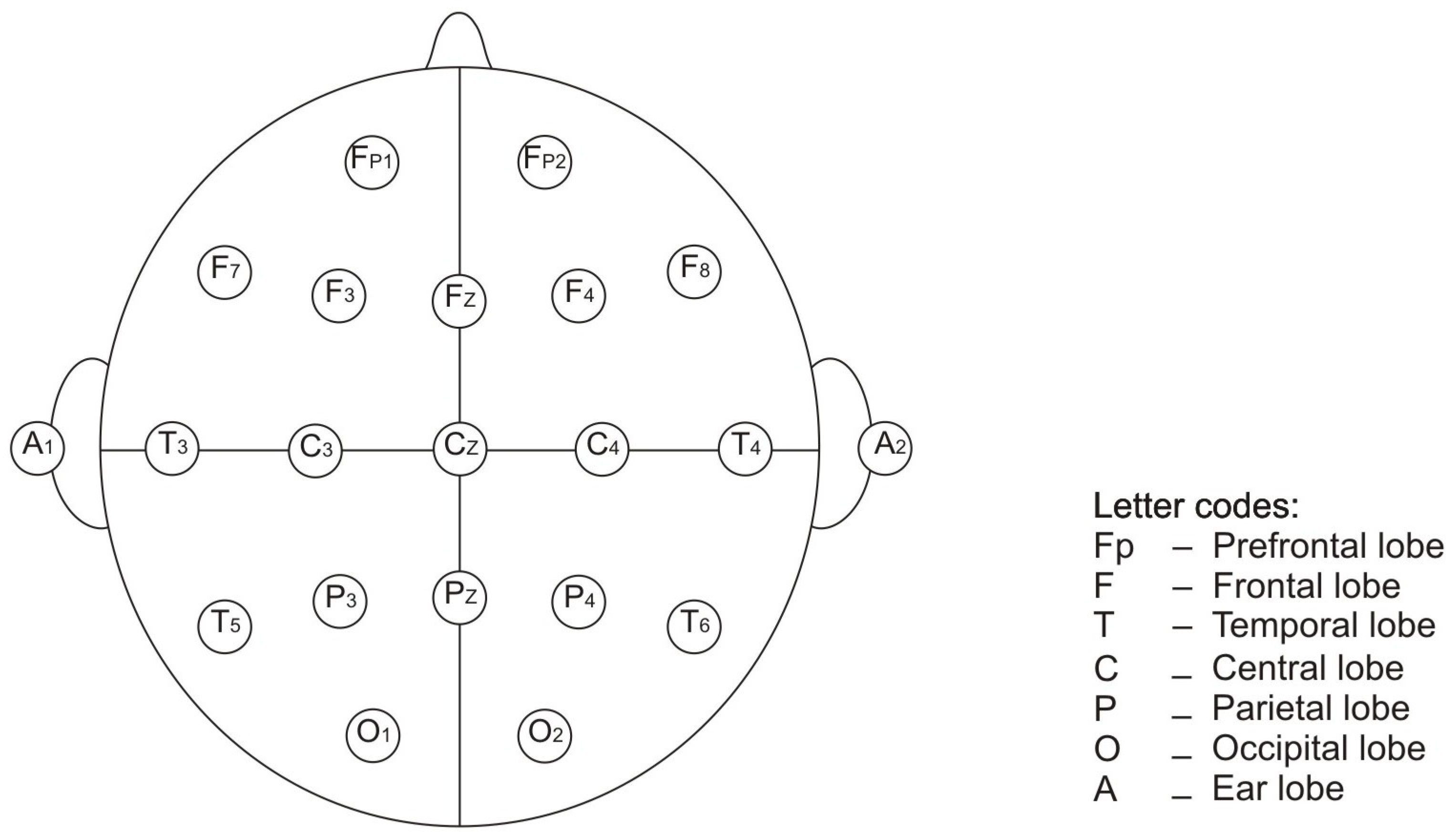
2.1. EEG Signals
2.2. EEG Signal Features
2.3. Amthauer Intelligence Structure Test
- IQ1—completion of sentences, logical thinking, language skills;
- IQ2—word exception, ability to abstract;
- IQ3—verbal analogies, combinatorial abilities;
- IQ4—conceptualization, ability for abstract verbal thinking;
- IQ5—calculations, mathematical abilities;
- IQ6—number series completion, ability to operate with numbers and inductive thinking;
- IQ7—figure detecting, combinatorial abilities;
- IQ8—identification of cubes, spatial imagination;
- IQ9—remembering words, ability for short-term storage of information.
3. Materials and Methods
3.1. Principal Component Regression
3.2. Data Description and Model Structure
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustafal, M.; Taib, M.N.; Lias, S.; Murat, Z.H.; Sulaiman, N. AEEG spectrogram classification employing ANN for IQ application. In Proceedings of the 2013 the International Conference on Technological Advances in Electrical, Electronics and Computer Engineering (TAEECE), Konya, Turkey, 9–11 May 2013; pp. 199–203. [Google Scholar]
- Mashiri, D. The relationship between projected IQ from QEEG and neurocognitive ability. New Voices Psychol. 2014, 10, 91–100. [Google Scholar] [CrossRef]
- Anoor, M.M.; Anwar, K.K.H.; Ali, M.S.A.M.; Jahidin, A.H. Classification of students’ IQ level using EEG-based intelligence classifier model. J. Fundam. Appl. Sci. 2017, 9, 684–694. [Google Scholar] [CrossRef]
- Luo, S.; Chen, R.; Yang, Z.; Li, K. Intelligence level might be predicted by the characteristics of EEG signals at specific frequencies and brain regions. J. Mech. Med. Biol. 2021, 21, 2140047. [Google Scholar] [CrossRef]
- Thatcher, R.W.; Palmero-Soler, E.; North, D.M.; Biver, C.J. Intelligence and eeg measures of information flow: Efficiency and homeostatic neuroplasticity. Sci. Rep. 2016, 6, 38890. [Google Scholar] [CrossRef]
- Anokhin, A.; Vogel, F. EEG alpha rhythm frequency and intelligence in normal adults. Intelligence 1996, 23, 1–14. [Google Scholar] [CrossRef]
- Firooz, S.; Setarehdan, S.K. IQ estimation by means of EEG-fNIRS recordings during a logical-mathematical intelligence test. Comput. Biol. Med. 2019, 110, 218–226. [Google Scholar] [CrossRef]
- Avdeenko, T.V.; Timofeeva, A.Y.; Murtazina, M.S. Modified Correlation-Based Feature Selection for Intelligence Estimation Based on Resting State EEG Data. Lect. Notes Comput. Sci. 2022, 13345, 289–300. [Google Scholar]
- Jahidin, A.H.; Taib, M.N.; Tahir, N.M.; Megat Ali, M.S.A.; Yassin, I.M.; Lias, S.; Isa, R.M.; Omar, W.R.W.; Fuad, N. Classification of intelligence quotient using EEG sub-band power ratio and ANN during mental task. In Proceedings of the 2013 IEEE Conference on Systems, Process & Control (ICSPC), Kuala Lumpur, Malaysia, 13–15 December 2013. [Google Scholar]
- Thatcher, R.W.; North, D.; Biver, C. EEG and intelligence: Relations between EEG coherence, EEG phase delay and power. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2005, 116, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Stankova, E.P.; Myshkin, I.Y. Association between individual EEG characteristics and the level of intelligence. Moscow Univ. Biol. Sci. Bull. 2016, 71, 256–261. [Google Scholar] [CrossRef]
- Kadam, S.T.; Dhaimodker, V.; Patil, M.M.; Reddy Edla, D.; Kuppili, V. EIQ: EEG based IQ test using wavelet packet transform and hierarchical extreme learning machine. J. Neurosci. Methods 2019, 322, 71–82. [Google Scholar] [CrossRef]
- Ros Azamin, N.J.; Taib, M.N.; Jahidin, A.H.; Awang, D.S.; Megat Ali, M.S.A. IQ level prediction and cross-relational analysis with perceptual ability using EEG-based SVM classification model. IAES Int. J. Artif. Intell. 2019, 8, 436–443. [Google Scholar] [CrossRef]
- Zakharov, I.; Tabueva, A.; Adamovich, T.; Kovas, Y.; Malykh, S. Alpha Band Resting-State EEG Connectivity Is Associated with Non-verbal Intelligence. Front. Hum. Neurosci. 2020, 14, 10. [Google Scholar] [CrossRef]
- Sanei, S.; Chambers, J.A. EEG Signal Processing; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Nottage, J.F.; Horder, J. State-of-the-Art Analysis of High-Frequency (Gamma Range) Electroencephalography in Humans. Neuropsychobiology 2015, 72, 219–228. [Google Scholar] [CrossRef]
- Mironovova, M.; Bíla, J. Fast fourier transform for feature extraction and neural network for classification of electrocardiogram signals. In Proceedings of the 2015 Fourth International Conference on Future Generation Communication Technology (FGCT), Luton, UK, 29–31 July 2015; pp. 1–6. [Google Scholar]
- Jović, A.; Suć, L.; Bogunović, N. Feature extraction from electroencephalographic records using EEGFrame framework. In Proceedings of the 36th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 20–24 May 2013; pp. 965–970. [Google Scholar]
- Bao, F.S.; Liu, X.; Zhang, C. PyEEG: An open source Python module for EEG/MEG feature extraction. Comput. Intell. Neurosci. 2011, 2011, 406391. [Google Scholar] [CrossRef]
- Liew, S.H.; Choo, Y.H.; Low, Y.F.; Yusoh, Z.I.M.; Yap, T.B.; Muda, A.K. Comparing Features Extraction Methods for Person Authentication Using EEG Signals. In Pattern Analysis, Intelligent Security and the Internet of Things. Advances in Intelligent Systems and Computing; Abraham, A., Muda, A., Choo, Y.H., Eds.; Springer: Cham, Switzerland, 2015; Volume 355. [Google Scholar]
- Bowyer, S.M. Coherence a measure of the brain networks: Past and present. Neuropsychiatr. Electrophysiol. 2016, 2, 1. [Google Scholar] [CrossRef]
- Coemets, E.H.; Liimets, H.I. Intellectual Tasks—Series 730. Russian Version of the Amthauer’s Test Based on the Estonian Methodic; Novosibirsk NSU Publisher: Novosibirsk, Russia, 1973; 24p. [Google Scholar]
- Bair, E.; Hastie, T.; Paul, D.; Tibshirani, R. Prediction by supervised principal components. J. Am. Stat. Assoc. 2006, 101, 119–137. [Google Scholar] [CrossRef]
- Krstajic, D.; Buturovic, L.J.; Leahy, D.E.; Thomas, S. Cross-validation pitfalls when selecting and assessing regression and classification models. J. Cheminform. 2014, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.S.; Liang, Y.Z. Monte Carlo cross validation. Chemom. Intell. Lab. Syst. 2001, 56, 1–11. [Google Scholar] [CrossRef]
- Mehraram, R.; Kaiser, M.; Cromarty, R.; Graziadio, S.; O’Brien, J.T.; Killen, A.; Taylor, J.-P.; Peraza, L.R. Weighted network measures reveal differences between dementia types: An EEG study. Hum. Brain Mapp. 2020, 41, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Onnela, J.P.; Saramäki, J.; Kertész, J.; Kaski, K. Intensity and coherence of motifs in weighted complex networks. Phys. Rev. E 2005, 71, 065103. [Google Scholar] [CrossRef] [PubMed]
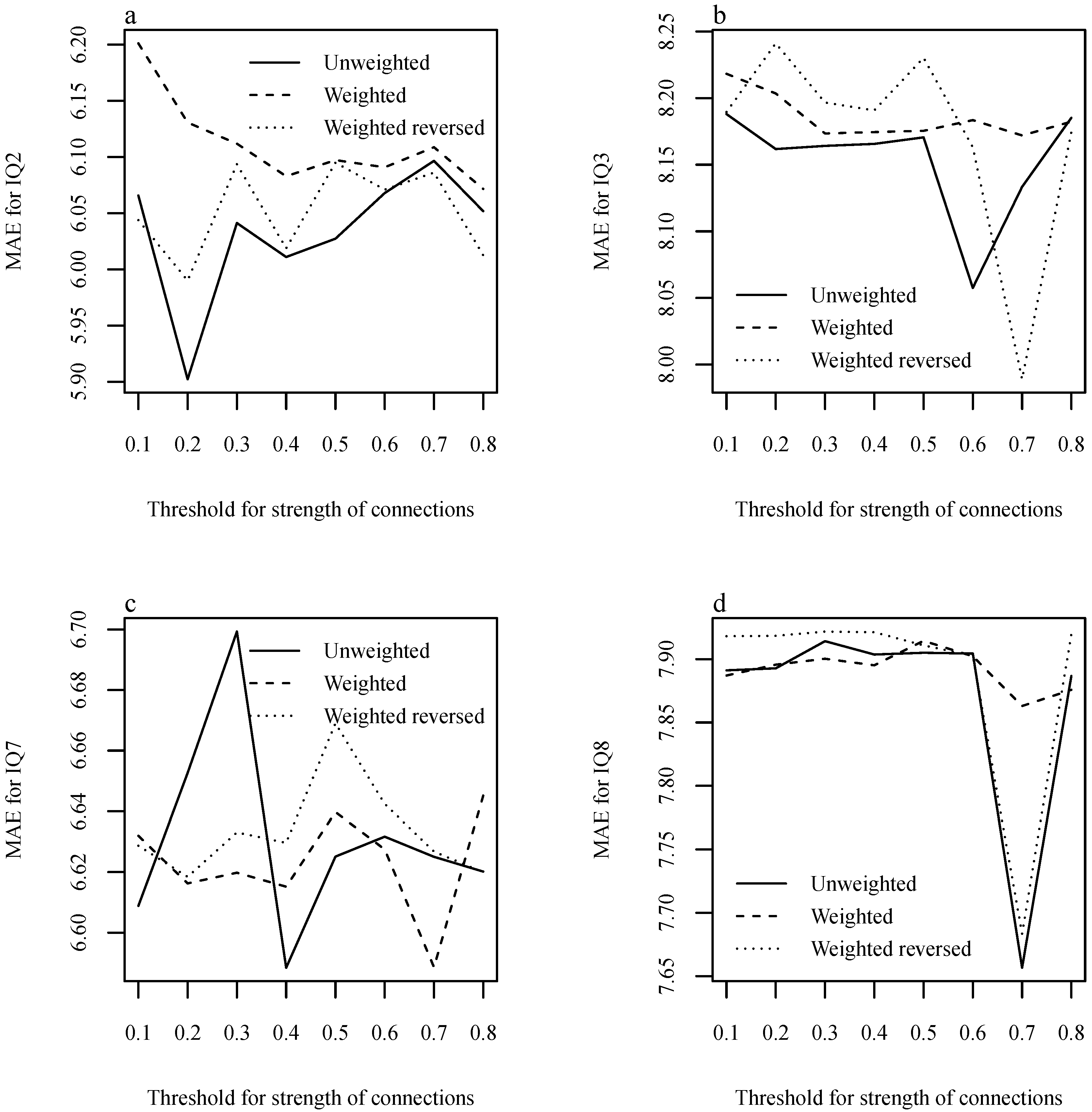
| IQ Component | The p-Value |
|---|---|
| IQ2 | 0.0354 |
| IQ3 | 0.5860 |
| IQ7 | 0.4334 |
| IQ8 | 0.6945 |
| IQ Component | Single-Channel | Multi-Channel |
|---|---|---|
| IQ2 | 5.663 (0.065) | 5.902 (0.128) |
| IQ3 | 7.763 (0.078) | 7.989 (0.153) |
| IQ7 | 6.860 (0.071) | 6.588 (0.135) |
| IQ8 | 7.809 (0.089) | 7.657 (0.165) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeenko, T.; Timofeeva, A.; Murtazina, M. Comparative Analysis of Feature Extraction Methods for Intelligence Estimation Based on Resting State EEG Data. Eng. Proc. 2023, 33, 25. https://doi.org/10.3390/engproc2023033025
Avdeenko T, Timofeeva A, Murtazina M. Comparative Analysis of Feature Extraction Methods for Intelligence Estimation Based on Resting State EEG Data. Engineering Proceedings. 2023; 33(1):25. https://doi.org/10.3390/engproc2023033025
Chicago/Turabian StyleAvdeenko, Tatiana, Anastasiia Timofeeva, and Marina Murtazina. 2023. "Comparative Analysis of Feature Extraction Methods for Intelligence Estimation Based on Resting State EEG Data" Engineering Proceedings 33, no. 1: 25. https://doi.org/10.3390/engproc2023033025
APA StyleAvdeenko, T., Timofeeva, A., & Murtazina, M. (2023). Comparative Analysis of Feature Extraction Methods for Intelligence Estimation Based on Resting State EEG Data. Engineering Proceedings, 33(1), 25. https://doi.org/10.3390/engproc2023033025






