Effective Corrosion Inhibition of Mild Steel in an Acidic Environment Using an Aqueous Extract of Macadamia Nut Green Peel Biowaste †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Inhibitor Preparation
2.1.2. Preparation of Specimens
2.2. Methods
Gasometric Method
3. Results
3.1. Influence of Inhibitor Concentration
3.2. Temperature Effect on Inhibition Performance of GPBW Extract
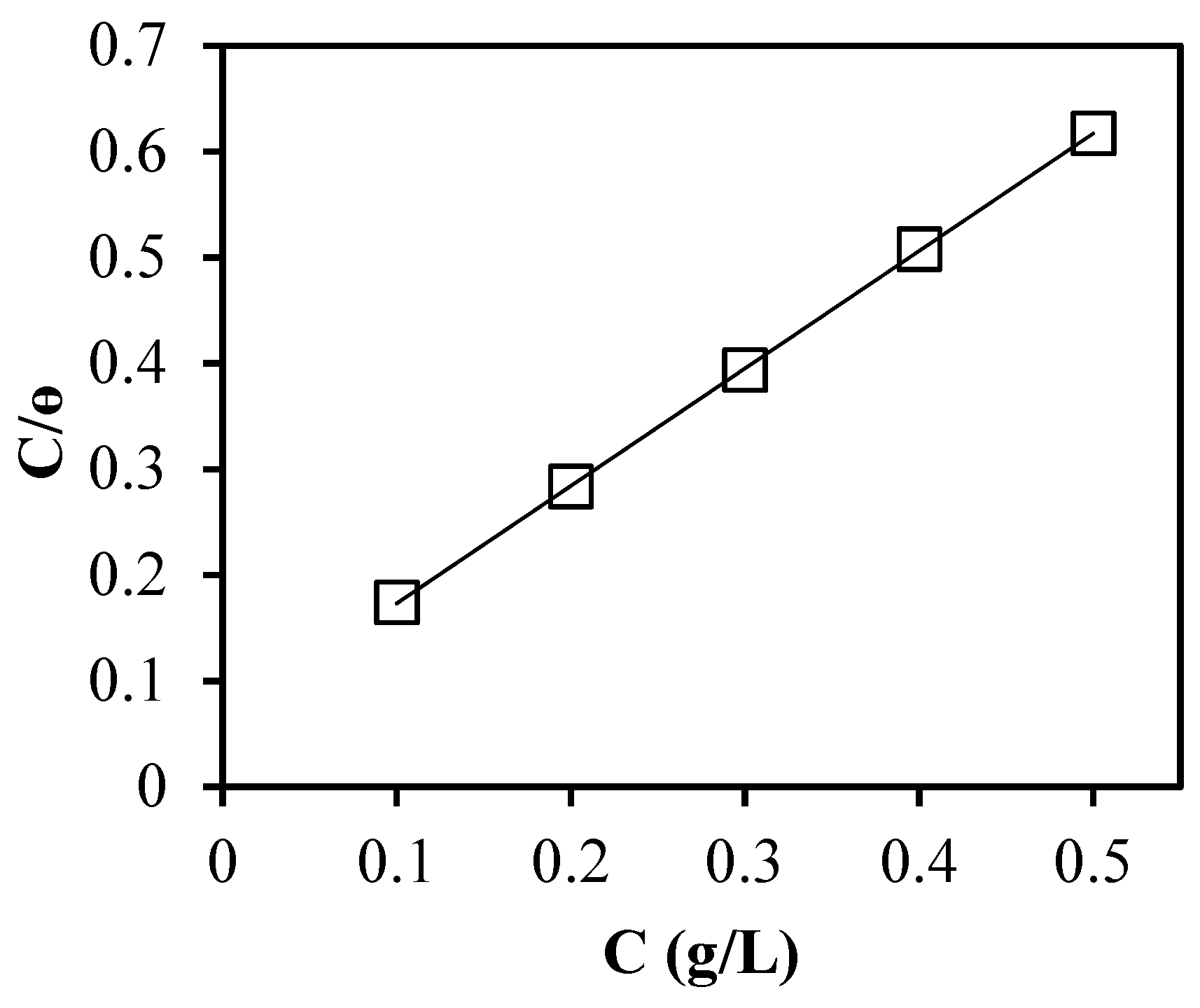
3.3. Adsorption Isotherms
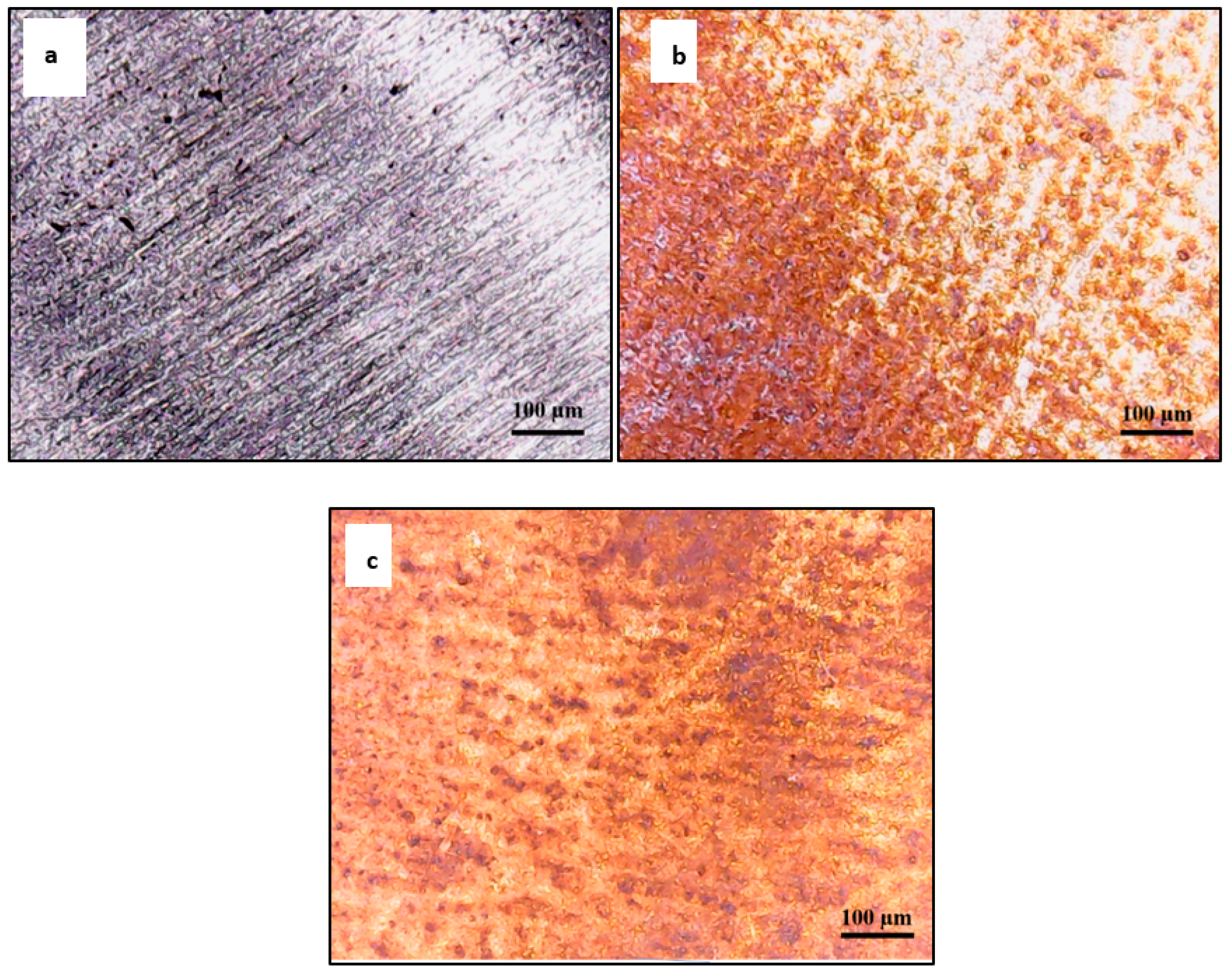
3.4. Optical Surface Examination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zehra, S.; Mobin, M.; Aslam, J. Chromates as corrosion inhibitors. In Inorganic Anticorrosive Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 251–268. [Google Scholar] [CrossRef]
- Fazal, B.R.; Becker, T.; Kinsella, B.; Lepkova, K. A review of plant extracts as green corrosion inhibitors for CO2 corrosion of carbon steel. NPJ Mater. Degrad. 2022, 6, 5. [Google Scholar] [CrossRef]
- Reza, N.; Akhmal, N.; Fadil, N.; Taib, M. A Review on Plants and Biomass Wastes as Organic Green Corrosion Inhibitors for Mild Steel in Acidic Environment. Metals 2021, 11, 1062. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2018, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Abbas, A.; Farooque, A.A.; Abbas, F.; Wang, X. Mitigation of Greenhouse Gas Emissions from Agricultural Fields through Bioresource Management. Sustainability 2022, 14, 5666. [Google Scholar] [CrossRef]
- Sarkar, S.; Skalicky, M.; Hossain, A.; Brestic, M.; Saha, S.; Garai, S.; Ray, K.; Brahmachari, K. Management of Crop Residues for Improving Input Use Efficiency and Agricultural Sustainability. Sustainability 2020, 12, 9808. [Google Scholar] [CrossRef]
- Dailey, A.; Vuong, Q.V. Optimization of Aqueous Extraction Conditions for Recovery of Phenolic Content and Antioxidant Properties from Macadamia (Macadamia tetraphylla) Skin Waste. Antioxidants 2015, 4, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Mwakalesi, A. Corrosion Inhibition of Mild Steel in Sulphuric Acid Solution with Tetradenia riparia Leaves Aqueous Extract: Kinetics and Thermodynamics. Biointerface Res. Appl. Chem. 2023, 13, 32. [Google Scholar] [CrossRef]
- Philip, J.Y.N.; Buchweshaija, J.; Mwakalesi, A. Corrosion Inhibition of Amino Pentadecylphenols (APPs) Derived from Cashew Nut Shell Liquid on Mild Steel in Acidic Medium. Mater. Sci. Appl. 2016, 7, 7. [Google Scholar] [CrossRef]
- Li, X.-L.; Xie, B.; Lai, C.; Feng, J.-S.; Liu, X.-Q.; Chen, L.; Yang, Y.-G.; Ji, R.-W.; He, J.-Y.; Li, W.; et al. Adsorption and corrosion inhibition performance of two planar rigid pyridinecarboxaldehyde-based double Schiff bases for mild steel in HCl solution: Experimental and computational investigations. J. Mol. Liq. 2022, 355, 118926. [Google Scholar] [CrossRef]
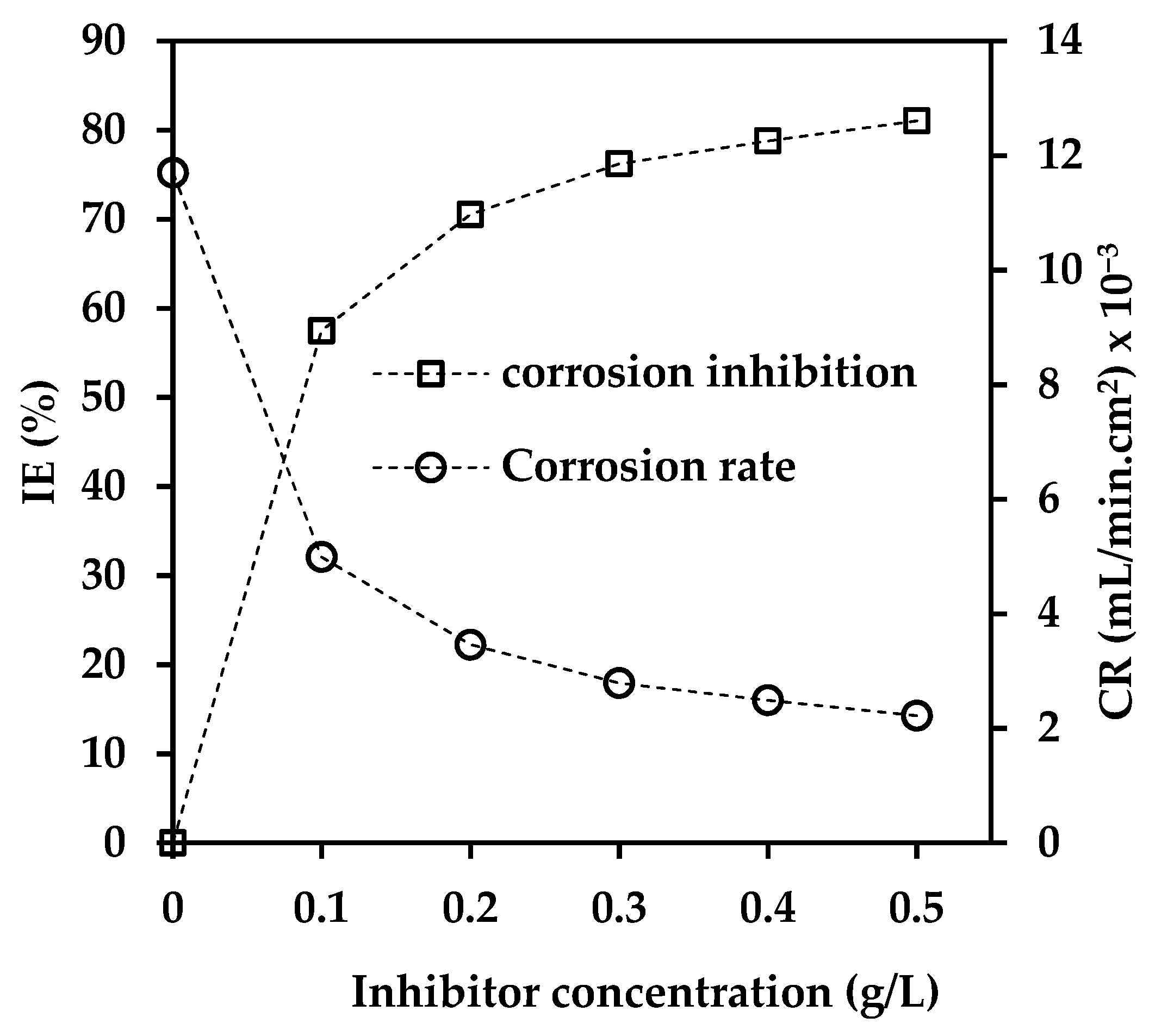

| Concentration (g/L) | CR (mL/min. cm2) × 10−3 | IE (%) | θ |
|---|---|---|---|
| 0 | 11.7 | 0 | 0 |
| 0.1 | 4.99 | 57 | 0.57 |
| 0.2 | 3.46 | 71 | 0.71 |
| 0.3 | 2.79 | 76 | 0.76 |
| 0.4 | 2.49 | 79 | 0.79 |
| 0.5 | 2.22 | 81 | 0.81 |
| Adsorption Isotherm | Relationship Plots | R2 |
|---|---|---|
| Langmuir | C/θ versus C | 0.9991 |
| Freundlich | log θ versus log C | 0.9633 |
| Frumkin | In (θ/C (1 − θ)) versus θ | 0.9742 |
| Temkin | θ versus log C | 0.9791 |
| Flory-Huggins | log θ/C versus (1 − θ) | 0.9596 |
| Inhibitor | Slope | Kads(L/g) | R2 | ∆G°ads (kJ/mol) |
|---|---|---|---|---|
| GPBW | 1.110 | 16.103 | 0.999 | −23.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwakalesi, A.J.; Nyangi, M. Effective Corrosion Inhibition of Mild Steel in an Acidic Environment Using an Aqueous Extract of Macadamia Nut Green Peel Biowaste. Eng. Proc. 2023, 31, 41. https://doi.org/10.3390/ASEC2022-13804
Mwakalesi AJ, Nyangi M. Effective Corrosion Inhibition of Mild Steel in an Acidic Environment Using an Aqueous Extract of Macadamia Nut Green Peel Biowaste. Engineering Proceedings. 2023; 31(1):41. https://doi.org/10.3390/ASEC2022-13804
Chicago/Turabian StyleMwakalesi, Alinanuswe J., and Magori Nyangi. 2023. "Effective Corrosion Inhibition of Mild Steel in an Acidic Environment Using an Aqueous Extract of Macadamia Nut Green Peel Biowaste" Engineering Proceedings 31, no. 1: 41. https://doi.org/10.3390/ASEC2022-13804
APA StyleMwakalesi, A. J., & Nyangi, M. (2023). Effective Corrosion Inhibition of Mild Steel in an Acidic Environment Using an Aqueous Extract of Macadamia Nut Green Peel Biowaste. Engineering Proceedings, 31(1), 41. https://doi.org/10.3390/ASEC2022-13804





