A Review on the Recent Developments in Passive Plasma Separators and Lab-on-Chip Microfluidic Devices †
Abstract
1. Introduction
2. Plasma Separation Techniques
2.1. Force-Driven Active Plasma Separation
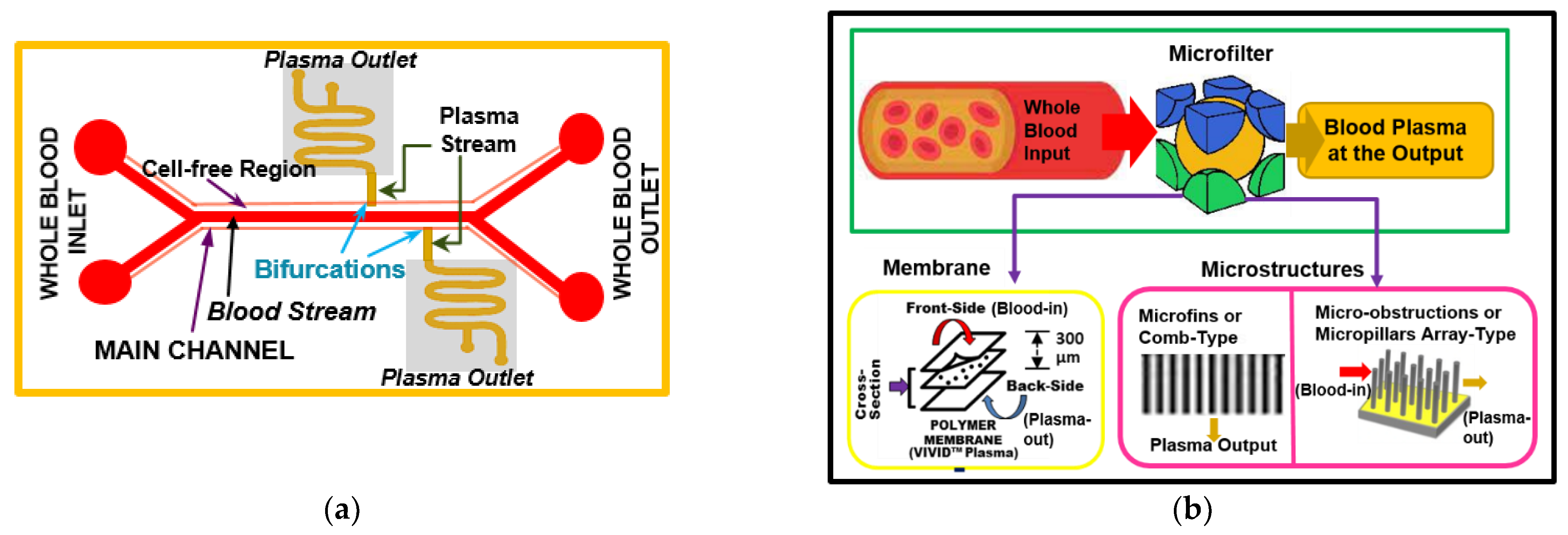
2.2. Self-Driven Passive Plasma Separation
3. Passive Lab-on-Chip Plasma Separation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| COVID 19 | Corona Virus Disease 2019 |
| SARS-CoV2 | Severe Acute Respiratory Syndrome Coronavirus2 |
| CHIKV | Chickengunya Virus |
| PDMS | Polydimethylsiloxane |
| PMMA | Polymethylmethacrylate |
| DLP | Digital Light processing |
| SLA | Stereolithography |
| SU 8 | Epoxy based Negative Photoresist |
References
- Eke, C.S.; Jammeh, E.; Li, X.; Carroll, C.; Pearson, S.; Ifeachor, E. Early Detection of Alzheimer’s Disease with Blood Plasma Proteins Using Support Vector Machines. IEEE J. Biomed. Health Informatics 2020, 25, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kazi, R.N. Early detection of kidney function in diabetic kidney disease: An approach to prevent end stage renal disease. J. Interv. Nephrol. 2018, 1, 15–18. [Google Scholar]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Rainer, T.; Wong, K.S.L.; Lam, W.; Yuen, E.; Lam, N.Y.; Metreweli, C.; Lo, Y.D. Prognostic Use of Circulating Plasma Nucleic Acid Concentrations in Patients with Acute Stroke. Clin. Chem. 2003, 49, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.S.; Vitorino, B.L.F.; Coelho, H.C.; Menezes-Neto, A.; Santos, M.L.S.; Campos, F.M.F.; Brito, C.F.; Fontes, C.; Lacerda, M.V.; Carvalho, L.H. Plasma Circulating Nucleic Acids Levels Increase According to the Morbidity of Plasmodium vivax Malaria. PLoS ONE 2011, 6, e19842. [Google Scholar] [CrossRef]
- Rhee, M.K.; Ho, Y.-L.; Raghavan, S.; Vassy, J.L.; Cho, K.; Gagnon, D.; Staimez, L.R.; Ford, C.N.; Wilson, P.W.F.; Phillips, L.S. Random plasma glucose predicts the diagnosis of diabetes. PLoS ONE 2019, 14, e0219964. [Google Scholar] [CrossRef]
- Wagner, J. Free DNA–new potential analyte in clinical laboratory diagnostics? Biochem. Medica 2012, 22, 24–38. [Google Scholar] [CrossRef]
- Benjamin, R.J.; McLaughlin, L.S. Plasma components: Properties, differences, and uses. Transfusion 2012, 52, 9S–19S. [Google Scholar] [CrossRef]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Kalmokoff, M.L.; Koval, S.F.; Jarrell, K.F. Relatedness of the flagellins from methanogens. Arch. Microbiol. 1992, 157, 481–487. [Google Scholar] [CrossRef]
- Cripps, C.M. Rapid method for the estimation of plasma haemoglobin levels. J. Clin. Pathol. 1968, 21, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nunna, B.; Talukder, N.; Etienne, E.; Lee, E. Blood Plasma Self-Separation Technologies during the Self-Driven Flow in Microfluidic Platforms. Bioengineering 2021, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.K.; Eriksen, K.B.; Christensen, M.L. Particle Track and Trace during Membrane Filtration by Direct Observation with a High Speed Camera. Membranes 2020, 10, 68. [Google Scholar] [CrossRef]
- Mateen, S.A.; Bhole, K.S. A review on microfluidic devices for separation of blood constituents. IOP Conf. Ser. Mater. Sci. Eng. 2020, 810, 012024. [Google Scholar] [CrossRef]
- Amasia, M.; Madou, M. Large-volume centrifugal microfluidic device for blood plasma separation. Bioanalysis 2010, 2, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Rafeie, M.; Zhang, J.; Asadnia, M.; Li, W.; Warkiani, M.E. Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation. Lab Chip 2016, 16, 2791–2802. [Google Scholar] [CrossRef]
- Civelekoglu, O.; Frazier, A.B.; Sarioglu, A.F. The Origins and the Current Applications of Microfluidics-Based Magnetic Cell Separation Technologies. Magnetochemistry 2022, 8, 10. [Google Scholar] [CrossRef]
- Yasukawa, T.; Yamada, J.; Shiku, H.; Matsue, T.; Suzuki, M. Microfluidic Separation of Blood Cells Based on the Negative Dielectrophoresis Operated by Three Dimensional Microband Electrodes. Micromachines 2020, 11, 833. [Google Scholar] [CrossRef]
- Nair, K.P.P.R.; Veettil, T.C.P.; Wood, B.R.; Paul, D.; Alan, T. Haemoprocessor: A Portable Platform Using Rapid Acoustically Driven Plasma Separation Validated by Infrared Spectroscopy for Point-of-Care Diagnostics. Biosensors 2022, 12, 119. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Wu, Z.-Q.; Wang, K.; Zhu, J.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Gravitational Sedimentation Induced Blood Delamination for Continuous Plasma Separation on a Microfluidics Chip. Anal. Chem. 2012, 84, 3780–3786. [Google Scholar] [CrossRef]
- Garcia-Rey, S.; Nielsen, J.B.; Nordin, G.P.; Woolley, A.T.; Basabe-Desmonts, L.; Benito-Lopez, F. High-Resolution 3D Printing Fabrication of a Microfluidic Platform for Blood Plasma Separation. Polymers 2022, 14, 2537. [Google Scholar] [CrossRef] [PubMed]
- Chien, W.; Zhang, Z.; Gompper, G.; Fedosov, D.A. Deformation and dynamics of erythrocytes govern their traversal through microfluidic devices with a deterministic lateral displacement architecture. Biomicrofluidics 2019, 13, 044106. [Google Scholar] [CrossRef]
- Rodríguez-Villarreal, A.I.; Arundell, M.; Carmona, M.; Samitier, J. High flow rate microfluidic device for blood plasma separation using a range of temperatures. Lab Chip 2009, 10, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Namgung, B.; Tan, J.K.S.; Wong, P.A.; Park, S.-Y.; Leo, H.L.; Kim, S. Biomimetic Precapillary Flow Patterns for Enhancing Blood Plasma Separation: A Preliminary Study. Sensors 2016, 16, 1543. [Google Scholar] [CrossRef]
- Lopresti, F.; Keraite, I.; Ongaro, A.E.; Howarth, N.M.; La Carrubba, V.; Kersaudy-Kerhoas, M. Engineered Membranes for Residual Cell Trapping on Microfluidic Blood Plasma Separation Systems: A Comparison between Porous and Nanofibrous Membranes. Membranes 2021, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, J.; Beebe, D.J. In situ fabricated porous filters for microsystems. Lab Chip 2003, 3, 62–66. [Google Scholar] [CrossRef]
- Andersson, H.; van der Wijngaart, W.; Enoksson, P.; Stemme, G. Micromachined flow-through filter-chamber for chemical reactions on beads. Sens. Actuators B Chem. 2000, 67, 203–208. [Google Scholar] [CrossRef]
- Zheng, S.; Lin, H.; Liu, J.-Q.; Balic, M.; Datar, R.; Cote, R.J.; Tai, Y.-C. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 2007, 1162, 154–161. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Pinho, D.; Lima, R.A.; Minas, G. A Passive Microfluidic Device Based on Crossflow Filtration for Cell Separation Measurements: A Spectrophotometric Characterization. Biosensors 2018, 8, 125. [Google Scholar] [CrossRef]
- Wang, Y.; Keller, K.; Cheng, X. Tangential Flow Microfiltration for Viral Separation and Concentration. Micromachines 2019, 10, 320. [Google Scholar] [CrossRef]
- Park, S.; Shabani, R.; Schumacher, M.; Kim, Y.-S.; Bae, Y.M.; Lee, K.-H.; Cho, H.J. On-chip whole blood plasma separator based on microfiltration, sedimentation and wetting contrast. Microsyst. Technol. 2015, 22, 2077–2085. [Google Scholar] [CrossRef]
- Madadi, H.; Casals-Terré, J.; Mohammadi, M. Self-driven filter-based blood plasma separator microfluidic chip for point-of-care testing. Biofabrication 2015, 7, 025007. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.; Chung, D.R.; Kim, T.; Park, E.; Kang, M. A self-pressure-driven blood plasma-separation device for point-of-care diagnostics. Talanta 2022, 247, 123562. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, J.; Zhang, D.; Wang, Y.; Chen, M.; Weng, Z.; Wang, J.; Zeng, J.; Zhang, Y.; Zhang, S.; et al. High-Efficiency Plasma Separator Based on Immunocapture and Filtration. Micromachines 2020, 11, 352. [Google Scholar] [CrossRef]
- Maria, M.S.; Rakesh, P.E.; Chandra, T.S.; Sen, A.K. Capillary flow of blood in a microchannel with differential wetting for blood plasma separation and on-chip glucose detection. Biomicrofluidics 2016, 10, 054108. [Google Scholar] [CrossRef] [PubMed]

| Whole Blood Cells | Cell Type | Value |
|---|---|---|
| Above 500 nm | RBCs | 6–8 µm |
| (Whole Blood Cells) | WBCs | 10–18 µm |
| Bacteria | 0.5–5 µm | |
| Below 500 nm (Plasma Cells) | RNAs, Proteins and Viruses | 2 nm (t-RNA *) 100–200 nm (mRNA *) 3.8 × 15 nm (Albumin) |
| 10–35 nm (Globulins) 50–140 nm (SARS-CoV-2) | ||
| HIV (100 nm) HBV, HCV (40–80 nm) CHIK-V (70nm) |
| Property | Whole Blood | Blood Plasma |
|---|---|---|
| Fluid TypeSpecific Gravity | Non-Newtonian1.052–1.056 | Newtonian 1.022–1.026 |
| Dynamic Viscosity | 3.5–5 cP@ γ * > 200 s−1 | 1.2–1.3 cP |
| Fluid Density | 1125 kg/m3 | 1025 kg/m3 |
| Cell size range | 2–8 µm | 20 nm to 140 nm |
| Fabrication Technology | Plasma Separator /Researcher/Year | Device Structure | Separation Technology | Efficiency/Analyte |
|---|---|---|---|---|
| Standard SU-8 Photolithography followed by PDMS Soft Lithography | On-chip whole blood plasma separator based on microfiltration, sedimentation and wetting contrast. Park et al. [31] (2015) | Patterning of PDMS to form a micropillar array employing soft lithography on the UV-developed and etched SU-8 mold for retarded flow and microfiltration. Patterning of glass via etching for developing micro-channels for plasma collection. | Retarded flow-assisted sedimentation and filtration of RBCs and WBCs, through array, while free flow wetting of plasma through the ethanol-treated microchannel. | 16 nL out of 15 µL of whole blood. Experimental model solution filtering out 4.5 µm of PS beads. |
| Self-driven filter-based blood plasma separator microfluidic chip for point-of-care testing. Madadi et al. [32] (2015) | The clogging delay caused by RBCs in a hydrophilic PDMS channel and the symmetric out- of-plane cross-flow filtration microchannel integrated micropillars (MIMPs), exploited to maximize the extracted plasma from undiluted blood. | Separation science, fluid dynamics, and blood rheology. Shear force acting on the main channel while the capillary forces are exerted on the plasma collection channel. | Extracted 0.1 µL of plasma from 5 µL of blood TSH qualitative testing employing diagnostic kit. | |
| SLA 3D Printing with a clear and colorless 3D printing material (Accura ClearVue™) | A self-pressure-driven blood plasma separation device for POC diagnosis. Kim et al. [33] (2022) | The separation device consists of a barrel and a plunger. The barrel holds the diluted whole blood sample. The plunger holds the glass fiber filter. Multiple LFA strip holder/house cover provided to hold rapid diagnostic kits. | Set of seals, self-pressurize the flow through the pored-matrix, binding the erythrocytes on the filter surface readily extracting plasma. | Multiple assay diagnostics
|
| CNC/CAD-CAM Micromachining of PMMA | High-efficiency plasma separator | Cup-shaped primary separation chamber (outer) loaded with anti-RBC-soaked acetate fiber | Acetate fiber matrix allows RBCs’ immunocapture. | Extracted 100 µL of Hemolysis-free 100% |
| Based on immunocapture and filtration. Su et al. [34] (2020) | pillar matrix (inner) and holds the blood sample. The final purification (bottom) chamber holds the separation membrane (VIVID™ GX), and connects the primary chamber and the plasma collection outlet. | GX- membrane allows size selection trapping of WBCs and platelets. | Pure plasma from 400 µL of whole blood sample. Quantitative PCR HBV testing. Non-protein biomarker glucose recovery rate is 100%± 0.73% | |
| Hybrid Technology | Capillary flow of blood in a micro-channel with differential wetting for blood plasma separation and on-chip glucose detection. Maria et al. [35] 2016 | DLP 3D printing, SU-8 photolithography followed with PDMS soft lithography. The main PDMS microchannels designed to be hydrophilic near the inlet side, and hydrophobic near the detection window. | Capillary-driven PDMS channel with dual wettability nature acts as a self-filter for plasma extraction exploiting differences in the viscosity and dynamic fluid velocities of the blood and plasma. | 450 nL pf plasma was extracted. Plasma recovery efficiency was 22.5%. on-chip detection of glucose. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatoon, S.; Ahmad, G. A Review on the Recent Developments in Passive Plasma Separators and Lab-on-Chip Microfluidic Devices. Eng. Proc. 2023, 31, 37. https://doi.org/10.3390/ASEC2022-13796
Khatoon S, Ahmad G. A Review on the Recent Developments in Passive Plasma Separators and Lab-on-Chip Microfluidic Devices. Engineering Proceedings. 2023; 31(1):37. https://doi.org/10.3390/ASEC2022-13796
Chicago/Turabian StyleKhatoon, Shamima, and Gufran Ahmad. 2023. "A Review on the Recent Developments in Passive Plasma Separators and Lab-on-Chip Microfluidic Devices" Engineering Proceedings 31, no. 1: 37. https://doi.org/10.3390/ASEC2022-13796
APA StyleKhatoon, S., & Ahmad, G. (2023). A Review on the Recent Developments in Passive Plasma Separators and Lab-on-Chip Microfluidic Devices. Engineering Proceedings, 31(1), 37. https://doi.org/10.3390/ASEC2022-13796





