Abstract
The bismuth film electrode (BiFE), which was first introduced in 2000 for electrochemical stripping analysis, is now widely used in electroanalytical laboratories worldwide. Numerous scientists have been inspired to conduct more research and broaden the understanding of the BiFE’s favourable electroanalytical performance, which is comparable to, or in some cases even exceeds, that of its mercury counterparts for the detection of heavy metal ions and selected organic compounds. Various types of bismuth-based paste electrodes, as well as in-situ and ex-situ prepared BiFE, have been presented in combination with potentiometric and voltammetric (stripping) protocols. Whereas the ex situ prepared electrodes must be moved from the modification solution to the measuring cell device and often need to display improved stability for several measurements, the ex-situ-prepared bismuth films require acceptable physical and chemical stability. In this study, we provided insight into the commencement of the formation of the bismuth film on a glassy carbon substrate electrode (GCE) when using a very low concentration of bismuth ions. We used our home-constructed AFM cell to fit in commercial working GCE, a platinum wire as the counter electrode, and an Ag/AgCl/NaCl (3 M) as the reference electrode.
1. Introduction
Bismuth (Bi) has a small carrier mass and a long electron free path at room temperature [1]. For potential uses of electronic quantum confinement effects in low-dimensional bismuth structures, these peculiar electronic characteristics are intriguing [2]. Owing to its physicochemical properties and its distinct environmentally friendly nature, Bi and its composites have been utilised in myriad applications (e.g., optoelectronic applications [3,4], electrocatalytic reduction [5], topological insulator [6], batteries [7,8,9], and heavy metal ions detection and removal [10,11,12]).
For electroanalytical applications, Bi-electrodes have emerged as a viable, alluring, and non-toxic substitute for conventional mercury-based electrodes since the introduction of bismuth film electrodes (BiFE) in 2000 [13]. Bi-based electrodes have characteristics that are most similar to those of mercury and have the advantage of being environmentally friendly with a favourable insensitivity towards dissolved oxygen. The carbon substrate in its various forms (such as glassy carbon, carbon paste, pencil lead, carbon fibre, and screen-printed carbon ink) appears to be the most suitable support for the Bi-film formation.
In situ AFM is a powerful technique, especially when combined with various electrochemical techniques [14,15,16,17,18], owing to its capability of monitoring the changes directly in testing solution with relatively high resolution without the need for sophisticated equipment, in comparison to the electron microscope when the sample under study is immersed in a solution.
In this work, we studied the commencement of the in situ formation of BiFE, i.e., the initiation of the bismuth film growth on a glassy carbon substrate using a very low concentration level of bismuth ions in acetate buffer solution. We used our home-constructed AFM cell to fit in the commercial working glassy carbon electrode to facilitate electrochemical measurements.
2. Materials and Methods
2.1. Materials
Bismuth standard solution (1000 mg/L Bi(III) in nitric acid) was purchased from Merck. Acetic acid (analytical grade) and platinum wire of 99.99% purity were purchased from Sigma Aldrich. A glassy carbon rotating disk electrode (GCE) with an outer diameter of 12 mm and disk diameter of 5 mm was purchased from Pin research.
2.2. Method
The exterior of GCE was machined to have a screw thread, so it could be attached to the electrochemical cell. The electrochemical cell, similar to our previously designed one [18], was manufactured in such a way that the working electrode’s position was machined to have a screw thread that the working electrode (GCE) could fit in. The cell was cleaned using an ultrasonic bath in acetone, isopropanol alcohol, and then Milli-Q water for 15 min each. Before attaching GCE to the electrochemical cell, the electrode was polished using alumina powder and nylon polishing pads; afterwards, it was rinsed with Milli-Q water. The platinum wire was used as the counter electrode, forming a circle within the inner rim of the cell, and the standard Ag/AgCl/NaCl (3M) (BASi) reference electrode was employed. The electrodes were positioned in the cell and connected to PalmSense 4 potentiostat.
2.3. AFM-Electrochemical Measurements
The GCE, Pt-wire, and reference electrodes were inserted in the electrochemical cell, and then the analyte (1 mg L−1 of Bi(III) in 0.5 M acetate buffer solution) was injected into the cell and scanned using Agilent 5500 AFM by operating it in AFM tapping mode. The electrode was scanned using a MikroMasch tip with a force constant of 5 N/m, 125 µm length, and coated with gold. The samples were scanned at a scanning rate of 1 HZ with a scanning area of 10 × 10 µm2.
3. Results and Discussion
After injecting the solution into the electrochemical cell, the tip was brought close enough to the electrode’s surface to scan it. Figure 1 shows selected AFM images of the GCE with the features formed on its surface. The left column encompasses the topography images, while the right column comprises the deflection images to additionally clarify the features at the surfaceGCE. As can be seen in Figure 1a, there are no bismuth deposits on the glassy carbon substrate surface except the scratches from the polishing process, which is confirmed in the corresponding deflection image (Figure 1b). A cathodic potential of −1.0 V vs. Ag/AgCl/NaCl (3M) was applied to GCE for a duration of 600 s. Then, the surface was scanned again at open circuit potential (OCP) at the same scanning area. The topography image in Figure 1c demonstrates the change in the surface and the formation of sparsely distributed nano-features resembling nanotubes, which is clearer in the deflection image (Figure 1d) when compared with Figure 1b. Next, to study the electrochemical manipulation of the so-formed nano-features, an anodic potential of +0.3 V vs. Ag/AgCl/NaCl (3M) was applied to GCE for 20 s, followed by AFM scanning of the same surface area, resulting in topography and deflection images, i.e., Figure 1e and Figure 1f, respectively. Upon the application of anodic potential, the bismuth nano-deposits were dissolved from the glassy carbon substrate. The application of the same cathodic potential accompanied by the same duration resulted in the formation of new nanostructured features (nanotubes), as depicted in Figure 1g,h. Moreover, the close inspection of the surface images revealed that the newly re-deposited bismuth structures did not start to grow at positions where the first-round bismuth film growth/dissolution occurred.
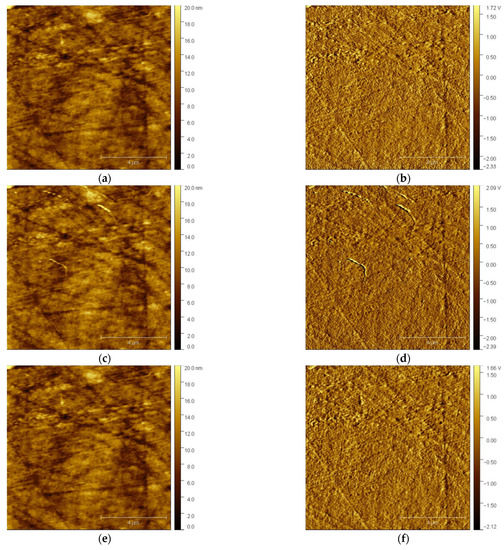
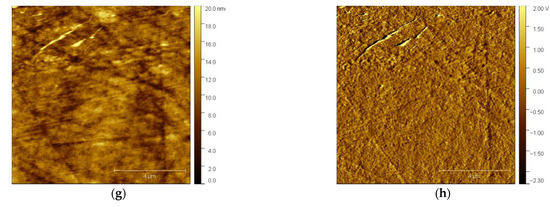
Figure 1.
In situ AFM images of GCE electrode surface in 0.5 M acetate buffer solution. The left column shows the topography, and the right column shows the deflection images: (a,b) fresh surface of GCE before electrochemical deposition; (c,d) after applying a cathodic potential of −1.0 V for 600 s; (e,f) after applying an anodic potential of +0.3 V for 20 s; (g,h) after applying a cathodic potential of −1.0 V for 600 s.
4. Conclusions
The combination of AFM with electrochemistry is a powerful approach for obtaining insight into the electrochemical processes at the conductive substrate surface. The formation/nucleation and dissolution of bismuth film with a low concentration of bismuth ions could be monitored directly in the solution using in situ AFM measurements. The commencement of the bismuth film formation was in the form of nanostructures resembling nanotubular features when using a cathodic potential of −1.0 V for 600 s. The electrochemically synthesised nanostructures were completely dissolved upon the application of an anodic potential of +0.3 V for 20 s and redeposited again using the same cathodic potential.
Author Contributions
Conceptualization, A.K. and S.B.H.; methodology, A.K. and S.B.H.; software, A.K.; validation, A.K.; investigation, A.K.; writing—original draft preparation, A.K.; writing—review and editing, A.K. and S.B.H.; funding acquisition, S.B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency (Research Programs P1-0034).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tian, M.; Wang, J.; Zhang, Q.; Kumar, N.; Mallouk, T.E.; Chan, M.H.W. Superconductivity and quantum oscillations in crystalline bi nanowire. Nano Lett. 2009, 9, 3196–3202. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, A.S.; Napolskii, K.S.; Skryabina, O.V.; Stolyarov, V.S.; Levin, E.E.; Egorov, S.V.; Eliseev, A.A.; Kasumov, Y.A.; Ryazanov, V.V.; Tsirlina, G.A. Bismuth nanowires: Electrochemical fabrication, structural features, and transport properties. Phys. Chem. Chem. Phys. 2020, 22, 14953–14964. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ren, X.; Li, Z.; Wang, H.; Huang, Z.; Qiao, H.; Tang, P.; Zhao, J.; Liang, W.; Ge, Y.; et al. Two-dimensional bismuth nanosheets as prospective photo-detector with tunable optoelectronic performance. Nanotechnology 2018, 29, 235201. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.D.; Shao, J.M.; Yang, G.W. Ultra-broadband and high-responsive photodetectors based on bismuth film at room temperature. Sci. Rep. 2015, 5, 12320. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, C.; Xia, B.; Jin, H.; Zheng, Y.; Qiao, S.-Z. Two-Dimensional Mosaic Bismuth Nanosheets for Highly Selective Ambient Electrocatalytic Nitrogen Reduction. ACS Catal. 2019, 9, 2902–2908. [Google Scholar] [CrossRef]
- Reis, F.; Li, G.; Dudy, L.; Bauernfeind, M.; Glass, S.; Hanke, W.; Thomale, R.; Schäfer, J.; Claessen, R. Bismuthene on a SiC substrate: A candidate for a high-temperature quantum spin Hall material. Science 2017, 357, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, C.; Zhang, S.; Hu, X.; Zhang, K.; Zhou, W.; Guo, S.; Xu, F.; Zeng, H. Ultrathin Bismuth Nanosheets for Stable Na-Ion Batteries: Clarification of Structure and Phase Transition by in Situ Observation. Nano Lett. 2019, 19, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.I.; Nakamoto, H.; Yamanaka, T.; Fukunaga, T.; Ogumi, Z.; Abe, T. Examination of Morphological Changes of Active Materials for Solution-Based Rechargeable Fluoride Shuttle Batteries Using in Situ Electro-chemical Atomic Force Microscopy Measurements. Chem. Mater. 2022, 34, 8280–8288. [Google Scholar] [CrossRef]
- Schorr, N.B.; Arnot, D.J.; Bruck, A.M.; Duay, J.; Kelly, M.; Habing, R.L.; Ricketts, L.S.; Vigil, J.A.; Gallaway, J.W.; Lambert, T.N. Rechargeable Alkaline Zinc/Copper Oxide Batteries. ACS Appl. Energy Mater. 2021, 4, 7073–7082. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Wang, X.; Zhao, D.; Rex, M.M.; Cho, H.J.; Lee, W.H. A novel nanoporous bismuth electrode sensor for in situ heavy metal detection. Electrochim. Acta 2019, 298, 440–448. [Google Scholar] [CrossRef]
- Zhang, D.; Xiang, Q. Nafion-Assisted Electrophoretic Deposition and Its Application in Bismuth Film Electrodes for Metal Ion Detection. Ind Eng Chem Res. 2021, 60, 11056–11062. [Google Scholar] [CrossRef]
- Franceschini, F.; Jagdale, P.; Bartoli, M.; Tagliaferro, A. Perspectives on the use of bismuth-based materials for sensing and removal of water pollutants. Curr. Opin. Environ. Sci. Health 2022, 26, 100345. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-Coated Carbon Electrodes for Anodic Stripping Voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Mihelčič, M.; Surca, A.K.; Kreta, A.; Gaberšček, M. Spectroscopical and Electrochemical Characterisation of a (3-Mercaptopropyl)trimethoxysilane-Based Protective Coating on Aluminium Alloy 2024. Croat. Chem. Acta 2017, 90, 169–175. [Google Scholar] [CrossRef]
- Rodošek, M.; Kreta, A.; Gaberšček, M.; Vuk, A. Ex situ IR reflection–absorption and in situ AFM electrochemical characterisation of the 1,2-bis(trimethoxysilyl)ethane-based protective coating on AA 2024 alloy. Corros. Sci. 2016, 102, 186–199. [Google Scholar] [CrossRef]
- Surca, A.K.; Rodošek, M.; Kreta, A.; Mihelčič, M.; Gaberšček, M. In Situ and Ex Situ Electrochemical Measurements: Spectroelectrochemistry and Atomic Force Microscopy. In Hybrid Organic-Inorganic Interfaces: Towards Advanced Functional Materialsl; Wiley: Hoboken, NJ, USA, 2017; pp. 793–837. [Google Scholar] [CrossRef]
- Kreta, A.; Rodošek, M.; Perše, L.S.; Orel, B.; Gaberšček, M.; Vuk, A. In situ electrochemical AFM, ex situ IR reflection–absorption and confocal Raman studies of corrosion processes of AA 2024-T3. Corros. Sci. 2016, 104, 290–309. [Google Scholar] [CrossRef]
- Kreta, A.; Gaberšček, M.; Muševič, I. Time-resolved in situ electrochemical atomic force microscopy imaging of the corrosion dynamics of AA2024-T3 using a new design of cell. J. Mater. Res. 2021, 36, 79–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).