1. Introduction
The degradation of active pharmaceutical ingredients (APIs) and the subsequent formation of degradation products affect the pharmaceutical quality of a medicinal product. Therefore, rapid and effective detection of adverse changes occurring in a medicinal product is very important and may be performed with the use of several novel techniques. Among these methods, the analysis of total directional hemispherical reflectance (THR) is of increasing importance. Previously, THR was used to distinguish falsified pharmaceuticals from originals [
1]. Analysis of reflectance and transmittance may be useful in the prediction of drug contents in tablets [
2]. In addition, some physical aspects of tablets, i.e., the hardness and porosity, may also be evaluated using near-infrared diffuse reflectance spectroscopy [
3].
We assumed that by using the method of hemispherical reflectance, adverse physicochemical changes in solid dosage drug forms can be detected.
The aim of the present study was to assess the total directional hemispherical reflectance (THR) of effervescent tablets containing magnesium and vitamin B6 during storage in ambient conditions.
2. Methods
2.1. Analyzed Tablets
Expired (n = 20; expiration date 04.2021) and unexpired (n = 20; expiration 03.2024) effervescent tablets containing magnesium and vitamin B6 (Zdrovit; Natur Produkt Pharma sp. z o. o., Ostrów Mazowiecka, Poland) were analyzed. Both tested types of tablets were evaluated in terms of diameter and thickness (measured using a caliper with an accuracy of 0.1 mm), and in terms of weight.
2.2. Images of the Tablets
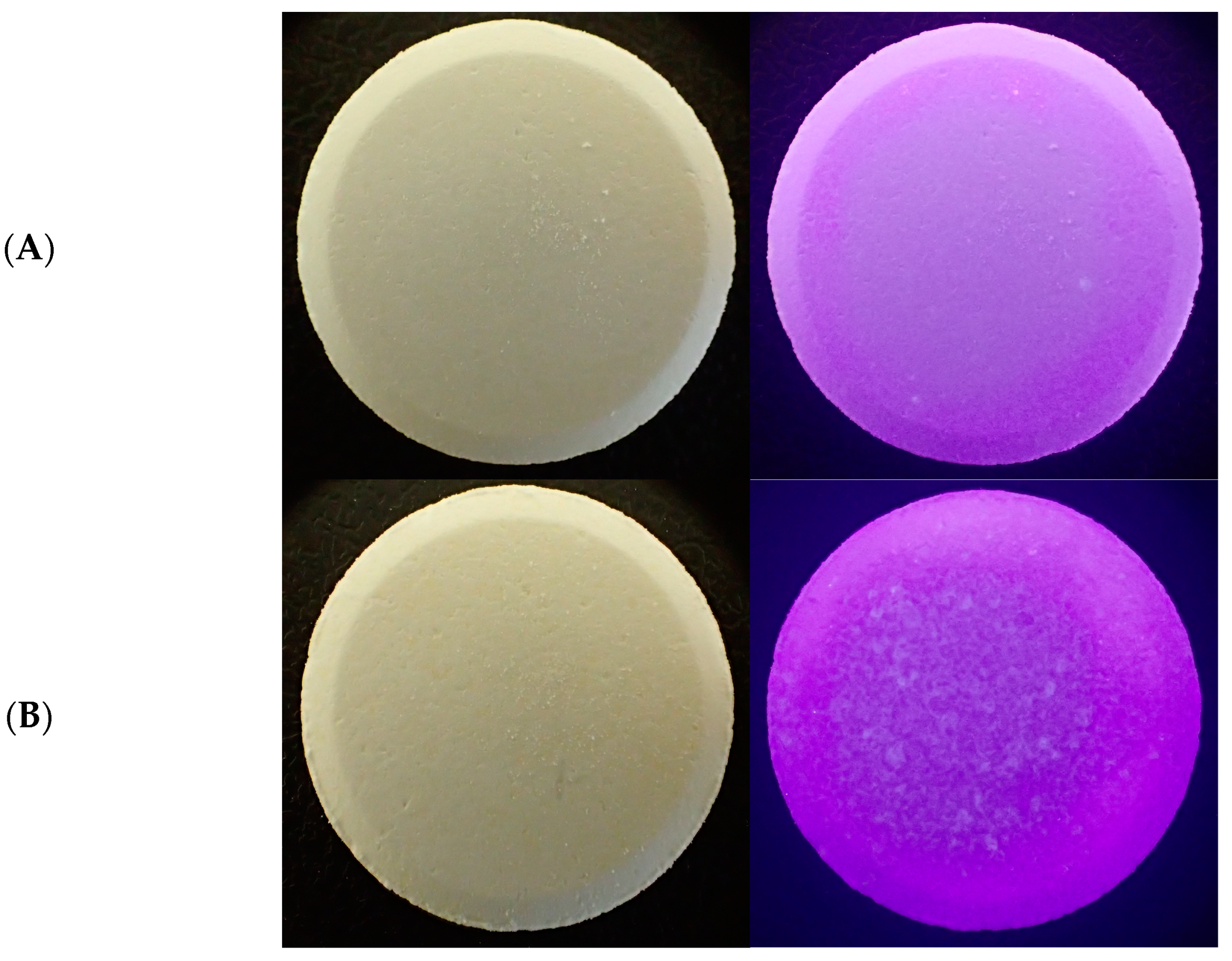
The external appearance of the effervescent tablets was also assessed using an Olympus Tough camera with a Dermlite attachment (Olympus Europa Se & Co. KG, Hamburg, Germany). Images under visible and UV lights were taken.
2.3. Reflectance Measurements
The measurements of THR were performed with the a SOC-410 Directional Hemispherical Reflectometer (USA) in seven wavelength bands from ultraviolet to near-infrared, i.e., 335–380 nm, 400–540 nm, 480–600 nm, 590–720 nm, 700–1100 nm, 1000–1700 nm, and 1700–2500 nm. The results for total reflectance were obtained with the beam at an angle of 20°. The mirror and diffuse calibration coupons were used to calibrate the reflectometer before the measurement. Each tablet was measured three times.
2.4. Statistical Analyses
Data were analyzed statistically using Statistica 13 software (StatSoft, Tulsa, OK, USA). The values of THR are presented as mean and standard deviation. Due to the preliminary stage of this study, we used a nonparametric Mann–Whitney U test to compare reflectance values between expired and unexpired effervescent tablets in all spectral bands. The value of p ≤ 0.05 was considered statistically significant.
3. Results and Discussion
The basic ingredients of effervescent tablets, in addition to the medicinal substance, are a soluble organic acid (usually citric, tartaric, malic, fumaric or adipic acid) and an alkali metal carbonate salt (sodium bicarbonate/carbonate or potassium bicarbonate/carbonate). These substances react in the presence of water to release carbon dioxide, resulting in the rapid release of the active pharmaceutical ingredient [
4,
5,
6]. Unfortunately, effervescent components are hygroscopic and moisture-labile, and storing tablets at temperatures above 25 °C accelerates the decomposition of bicarbonate to carbonate. Increasingly, this form provides a source of vitamins and/or minerals or other substances with nutritional or other physiological effects [
7,
8].
Solid oral dosage forms are commonly manufactured in batches, of which only randomly selected samples are tested by often time-consuming offline testing. The use of spectroscopic techniques allows for obtaining new spatial information about samples and additional quality control of samples from a given batch in a non-destructive, non-invasive, fast, and time-saving manner. Moreover, spectroscopic techniques and imaging techniques can be used not only during the entire production process but also during the storage of a pharmaceutical product, making it possible to correlate the data obtained from them with the data obtained using pharmacopeial stability assessment methods.
3.1. Characteristics of the Analyzed Types of Effervescent Tablets
Both analyzed types of effervescent tablets were cylindrical with flat top and bottom surfaces. The expired effervescent tablets showed some discoloration, impurities, or cracks (
Figure 1). Additionally, both types of tablets, expired and unexpired, were characterized by the amount of powder on the surface, which may suggest high friability. The mean thickness, diameter, and weight of the unexpired tablets were 5.66 mm, 25.20 mm, and 4.012 g, respectively. The mean thickness, diameter, and weight of the expired tablets were 5.73 mm, 25.23 mm, and 4.007 g, respectively.
3.2. Comparison of Expired and Unexpired Tablets in Terms of THR Value
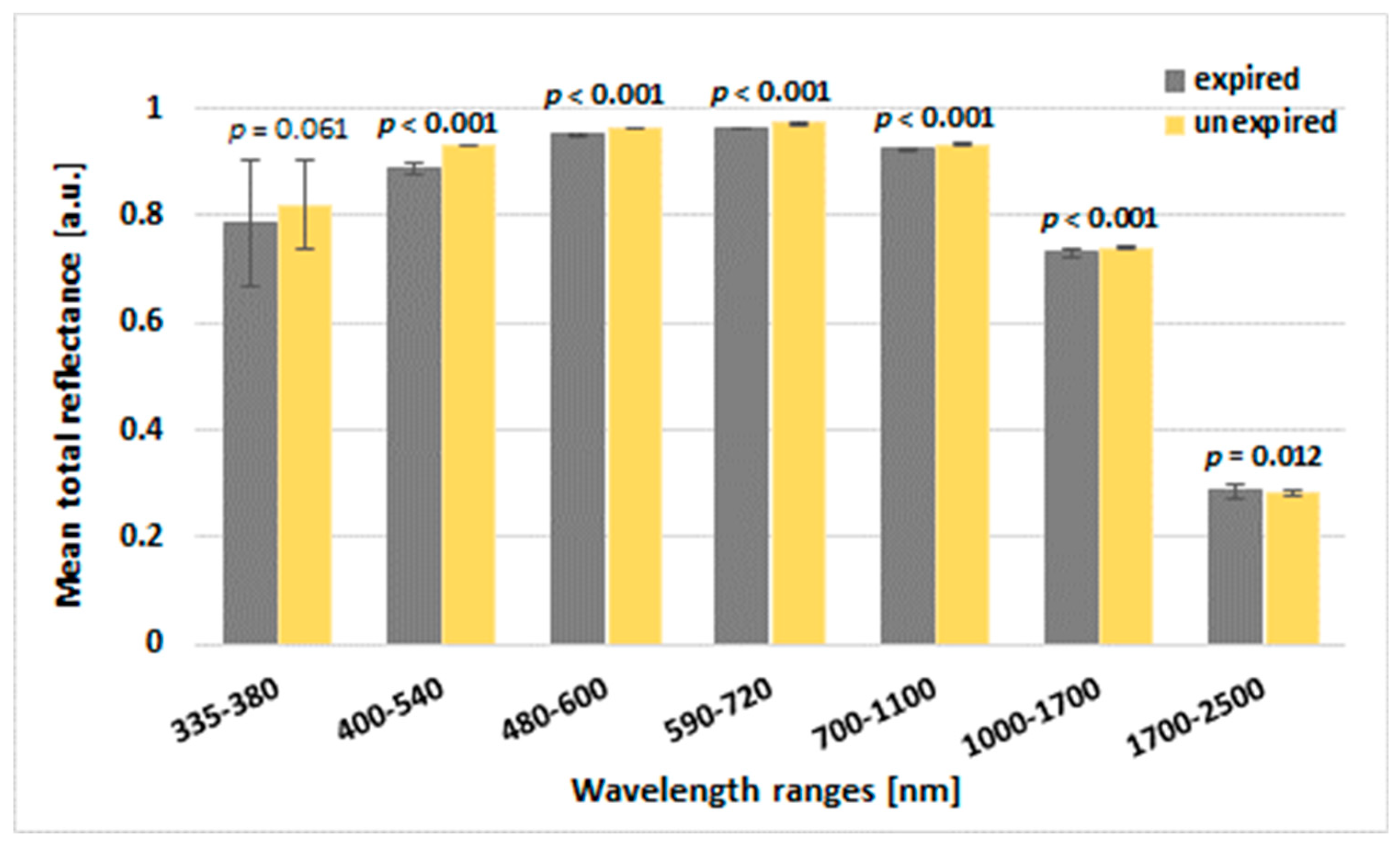
The mean THR was significantly higher within the ranges of 400–540 nm, 480–600, 590–720, 700–1100 nm, and 1000–1700 for the unexpired effervescent tablets compared to the expired tablets (
p < 0.001). In turn, a significantly higher value of THR for the range of 1700–2500 nm was observed for expired tablets than for unexpired tablets (
p = 0.012) (
Figure 2). Surprisingly, we observed significant differences in the mean THR between the top side and the bottom side of the tablets.
4. Conclusions
Lower values of THR for expired effervescent tablets compared to unexpired ones may be explained by the fact that some physicochemical changes occurred during the storage, and in this state, the reflectance of the light beam is lower since more light is transmitted inside the tablet.
The measurement of THR may be a rapid test for detecting adverse physicochemical changes in these tablets and can be used for a further detailed evaluation of API and related degradation products.
Author Contributions
Conceptualization, B.S.-H. and B.S.-M.; methodology, B.S.-H., B.S.-M., M.M. and P.D.; software, B.S.-H., B.S.-M., M.M. and P.D.; formal analysis, B.S.-H. and B.S.-M.; investigation, B.S.-H., B.S.-M., M.M. and P.D.; resources, B.S.-H. and B.S.-M.; data curation, B.S.-H., B.S.-M., M.M. and P.D.; writing—original draft preparation, B.S.-H., B.S.-M., M.M. and P.D.; writing—review and editing, B.S.-H. and B.S.-M.; visualization, B.S.-H.; supervision, B.S.-H.; project administration, B.S.-H.; funding acquisition, B.S.-H. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Medical University of Silesia in Katowice, Poland, within the project: PCN-1-058/K/2/O.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request in the Department of Basic Biomedical Science, School of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia in Katowice, Poland. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilczyński, S.; Koprowski, R.; Błońska-Fajfrowska, B. Directional reflectance analysis for identifying counterfeit drugs: Preliminary study. J. Pharm. Biomed. Anal. 2016, 124, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Forbess, R.A.; Ebube, N.K.; Chen, Y.; Rubinovitz, R.L.; Kemper, M.S.; Reier, G.E.; Wheatley, T.A.; Shukla, A.J. A comparison of reflectance and transmittance near-infrared spectroscopic techniques in determining drug content in intact tablets. Pharm. Dev. Technol. 2001, 6, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Donoso, M.; Kildsig, D.O.; Ghaly, E.S. Prediction of tablet hardness and porosity using near-infrared diffuse reflectance spectroscopy as a nondestructive method. Pharm. Dev. Technol. 2003, 8, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Siddaiah, M. Formulation and evaluation of effervescent tablets: A review. J. Drug Deliv. Ther. 2018, 8, 296–303. effervescent tablets. Adv. Pharm. Bull. 2013, 3, 217–225. [Google Scholar]
- Aslani, A.; Fattahi, F. Formulation, characterization and physicochemical evaluation of potassium citrate effervescent tablets. Adv. Pharm. Bull. 2013, 3, 217–225. [Google Scholar] [PubMed]
- Aslani, A.; Jahangiri, H. Formulation, characterization and physicochemical evaluation of ranitidine effervescent tablets. Adv. Pharm. Bull. 2013, 3, 315–322. [Google Scholar]
- Wegehaupt, F.J.; Lunghi, N.; Hogger, V.M.G.; Attin, T. Erosive potential of vitamin and vitamin+mineral effervescent tablets. Swiss Dent. J. 2016, 126, 457–465. [Google Scholar]
- Gurdogan Guler, E.B.; Bayrak, G.D.; Unsal, M.; Selvi Kuvvetli, S. Effect of pediatric multivitamin syrups and effervescent tablets on the surface microhardness and roughness of restorative materials: An in vitro study. J. Dent. Sci. 2021, 16, 311–317. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).