MIP-Based Screen-Printed Electrode for Irbesartan Sensing †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Prepolymeric Mixture Preparation and Modification of the Working Electrode Surface
3. Results
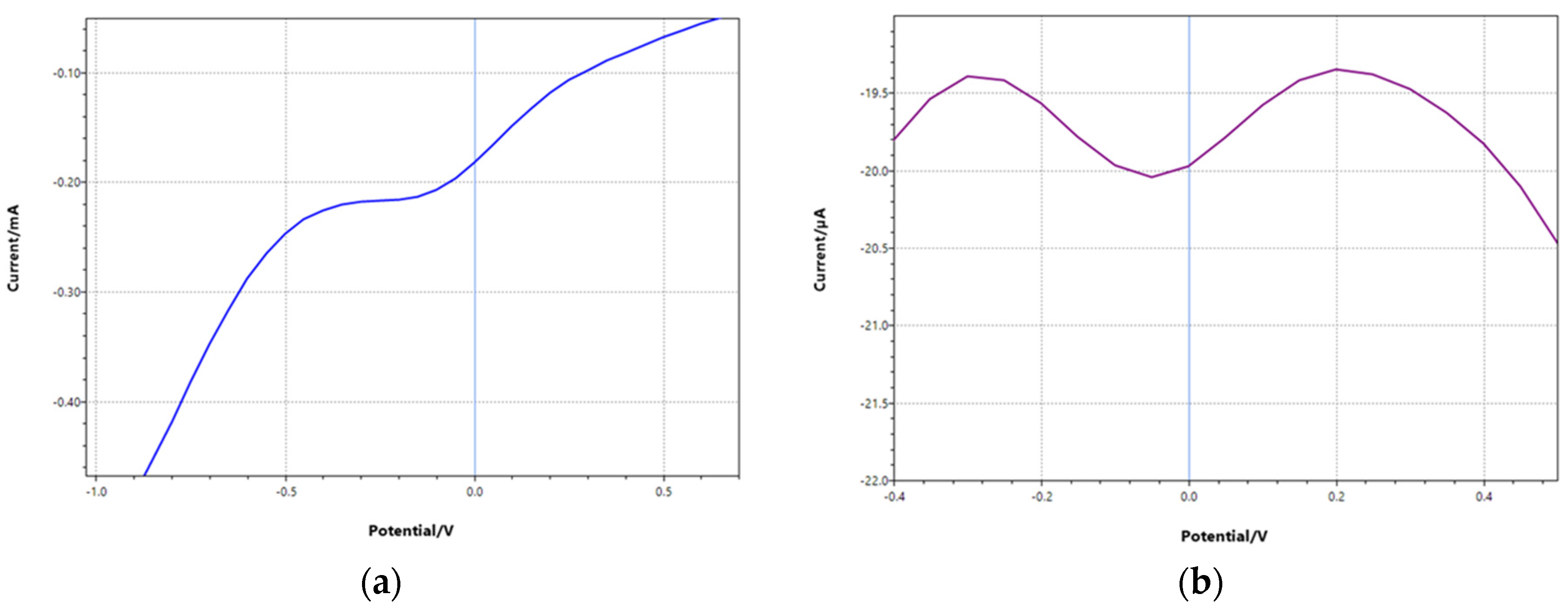
3.1. Polymer Characterization
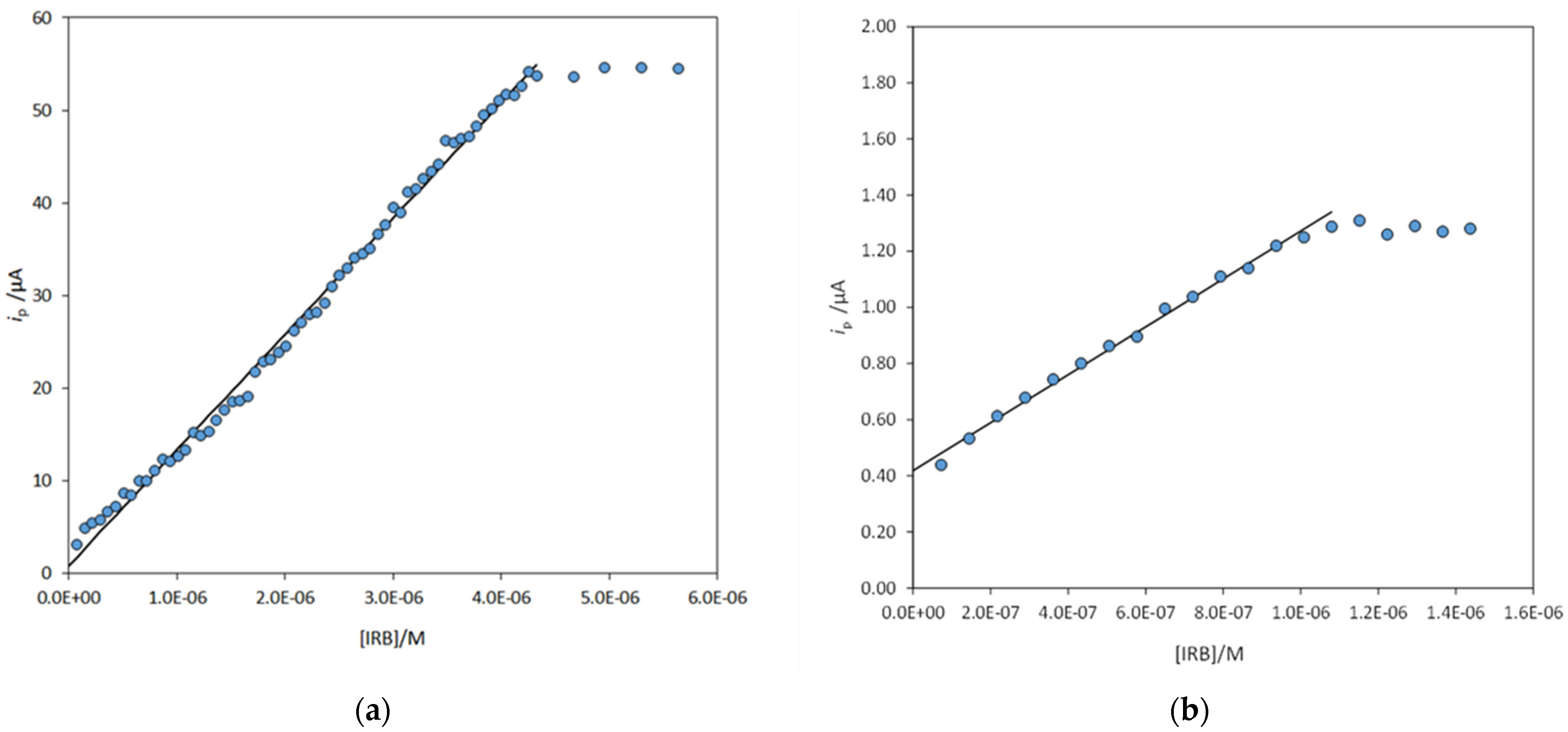
3.2. Electrode Functionalization and Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I.; et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Approccio Strategico dell’Unione Europea Riguardo all’Impatto Ambientale dei Farmaci. Available online: https://ec.europa.eu/transparency/regdoc/rep/1/2019/IT/COM-2019-128-F1-IT-MAIN-PART-1.PDF (accessed on 11 October 2022).
- Bayer, A.; Asner, R.; Schüssler, W.; Kopf, W.; Weiß, K.; Sengl, M.; Letzel, M. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ. Sci. Pollut. Res. 2014, 21, 10830–10839. [Google Scholar] [CrossRef] [PubMed]
- CastroI, G.; Rodríguez, I.; Ramil, M.; Cela, R. Selective determination of sartan drugs in environmental water samples by mixed-mode solid-phase extraction and liquid chromatography tandem mass spectrometry. Chemosphere 2019, 224, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Muszalska, I.; Sobczak, A.; Dołhań, A.; Jelińska, A. Analysis of Sartans: A Review. J. Pharm. Sci. 2014, 103, 2–28. [Google Scholar] [CrossRef] [PubMed]
- Rane, V.P.; Patil, K.R.; Sangshetti, J.N.; Yeole, R.D.; Shinde, D.B. Stability indicating LC method for simultaneous determination of irbesartan and hydrochlorothiazide in pharmaceutical preparations. J. Chromatogr. Sci. 2010, 48, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Piletska, E.V.; Guerreiro, A.R.; Whitcombe, M.J.; Piletsky, S.A. Influence of the Polymerization Conditions on the Performance of Molecularly Imprinted Polymers. Macromolecules 2009, 42, 4921–4928. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Alberti, G.; Amendola, V.; Pesavento, M.; Biesuz, R. Beyond the synthesis of novel solid phases: Review on modelling of sorption phenomena. Coord. Chem. Rev. 2012, 256, 28–45. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Agarwal, S.; Mishra, R.; Dwivedi, A. Electrochemical determination of antihypertensive drug irbesartan in pharmaceuticals. Anal. Biochem. 2011, 10, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Bozal, B.; Doğan-Topal, B.; Uslu, B.; Özkan, S.A.; Aboul-Enein, H.Y. Quantitative analysis of irbesartan in pharmaceuticals and human biological fluids by voltammetry. Anal. Lett. 2009, 42, 2322–2338. [Google Scholar]
- Palabıyık, İ.M.; Dogan, A.; Süslü, İ. Simultaneous Determination of Amlodipine and Irbesartan in their Pharmaceutical Formulations by Square-Wave Voltammetry. Comb. Chem. High Throughput Scree. 2022, 25, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Burak, D.; Emregul, E.; Emregul, K.C. Copper–Zinc Alloy Nanoparticle Based Enzyme-Free Superoxide Radical Sensing on a Screen-Printed Electrode. Talanta 2015, 134, 206–214. [Google Scholar]
- Pesavento, M.; Merli, D.; Biesuz, R.; Alberti, G.; Marchetti, S.; Milanese, C. A MIP-based low-cost electrochemical sensor for 2-furaldehyde detection in beverages. Anal. Chim. Acta 2021, 1142, 201–210. [Google Scholar] [CrossRef] [PubMed]


| Sensor | [IRB]/M Nominal | [IRB]/M Measured | Error % | Recovery % |
|---|---|---|---|---|
| SPC-MIP | 3.6 × 10−7 | 3.37(6) × 10−7 | −6.6 | 93.4 |
| SPC-bare | 3.6 × 10−7 | 1.2(1) × 10−7 | −66.5 | 33.5 |
| SPC-MIP | 1.4 × 10−6 | 1.3(2) × 10−6 | −7.4 | 92.6 |
| SPC-bare | 1.4 × 10−6 | 3.9(5) × 10−7 | −72.8 | 27.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovida, R.; Zanoni, C.; Alberti, G.; Magnaghi, L.R.; Biesuz, R. MIP-Based Screen-Printed Electrode for Irbesartan Sensing. Eng. Proc. 2023, 31, 22. https://doi.org/10.3390/ASEC2022-13836
Rovida R, Zanoni C, Alberti G, Magnaghi LR, Biesuz R. MIP-Based Screen-Printed Electrode for Irbesartan Sensing. Engineering Proceedings. 2023; 31(1):22. https://doi.org/10.3390/ASEC2022-13836
Chicago/Turabian StyleRovida, Riccardo, Camilla Zanoni, Giancarla Alberti, Lisa Rita Magnaghi, and Raffaela Biesuz. 2023. "MIP-Based Screen-Printed Electrode for Irbesartan Sensing" Engineering Proceedings 31, no. 1: 22. https://doi.org/10.3390/ASEC2022-13836
APA StyleRovida, R., Zanoni, C., Alberti, G., Magnaghi, L. R., & Biesuz, R. (2023). MIP-Based Screen-Printed Electrode for Irbesartan Sensing. Engineering Proceedings, 31(1), 22. https://doi.org/10.3390/ASEC2022-13836









