Reasons for High Adsorption Efficiencies in Lead Removal from Aquatic Solution †
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Low Melting Liquid Metal State
3.2. Post-Transition Metal and Electron Distribution
3.3. Amphoter/Amphoteric Structure
3.4. Adsorption/Biosorption Pre-Treatment Studies
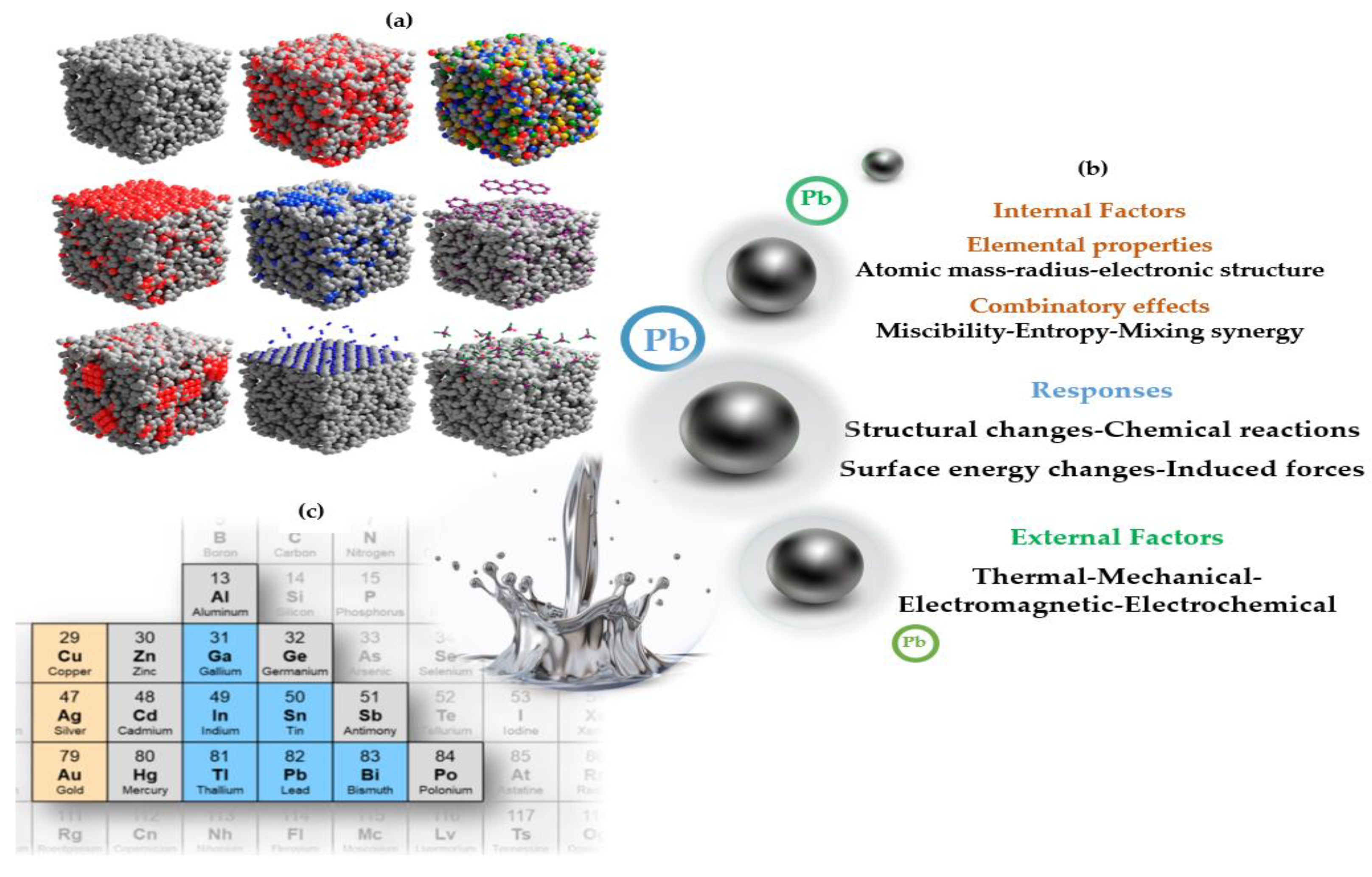
4. Discussion
- Active components (pectin, catechin, lignin, etc.) in the structures of adsorbents and biosorbents and functional groups (carboxyl:-O, amines: -NH, and hydroxyl: -OH) of these sorbents can show a strong interaction with Pb.
- The amphoteric/amphoteric nature of Pb may increase the removal rate.
- Another factor is that some of the physical properties of Pb (high density, molecular weight, etc.) are different from those of other metals.
- Being in the liquid metal group free two electron distribution can affect the adhesion to the surface of the sorbents in the adsorption mechanism.
- The nature of the sorbents, their amphoteric properties, and the modification stages explain the bonding and sorption mechanisms of Pb.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Bhunia, P. Environmental toxicants and hazardous contaminants: Recent advances in technologies for sustainable development. J. Hazard. Toxic. Radioact. Waste 2017, 21, 02017001. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Pourang, N.; Moradi, A.M. Removal of lead from aqueous solutions using three biosorbents of aquatic origin with the emphasis on the affective factors. Sci. Rep. 2022, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yuan, X.; Wu, Z.; Zeng, G.; Jiang, L.; Peng, X.; Li, H. Adsorption behavior and mechanism of Mg/Fe layered double hy-droxide with Fe3O4-carbon spheres on the removal of Pb (II) and Cu (II). J. Colloid Interface Sci. 2019, 536, 440–455. [Google Scholar] [CrossRef]
- Yousef, R.; Qiblawey, H.; El-Naas, M.H. Adsorption as a process for produced water treatment: A review. Processes 2020, 8, 1657. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Qu, J.; Meng, Q.; Lin, X.; Han, W.; Jiang, Q.; Wang, L.; Hu, Q.; Zhang, L.; Zhang, Y. Microwave-assisted synthesis of betacyclodextrin functionalized celluloses for enhanced removal of Pb (II) from water: Adsorptive performance and mechanism exploration. Sci. Total Environ. 2020, 752, 141854. [Google Scholar] [CrossRef]
- Wang, L.; Lei, T.; Ren, Z.; Jiang, X.; Yang, X.; Bai, H.; Wang, S. Fe3O4@PDA@MnO2 core-shell nanocomposites for sensitive elec-trochemical detection of trace Pb (II) in water. J. Electroanal. Chem. 2020, 864, 114065. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Li, N.; Tao, J.; Yan, B.; Cui, X.; Chen, G. Adsorption of lead from aqueous solution by biochar: A review. Clean Technol. 2022, 4, 39. [Google Scholar] [CrossRef]
- Dai, K.; Liu, G.; Xu, W.; Deng, Z.; Wu, Y.; Zhao, C.; Zhang, Z. Judicious fabrication of bifunctionalized graphene oxide/MnFe2O4 magnetic nanohybrids for enhanced removal of Pb (II) from water. J. Colloid Interface Sci. 2020, 579, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Hasan, M.M.; Islam, A.; Rahman, M.M.; Asiri, A.M.; Khaleque, M.A.; Sheikh, M.C. Offering an innovative composited material for effective lead (II) monitoring and removal from polluted water. J. Clean. Prod. 2019, 231, 214–223. [Google Scholar] [CrossRef]
- Cao, H.; Ma, X.; Wei, Z.; Tan, Y.; Chen, S.; Ye, T.; Yuan, M.; Yu, J.; Wu, X.; Yin, F.; et al. Behavior and mechanism of the adsorption of lead by an eco-friendly porous double-network hydrogel derived from keratin. Chemosphere 2022, 289, 133086. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, H.; Bahadır, T.; ¸Simsek, İ.; Tulun, Ş. An environmental and green process for Pb2+ pollution: An experimental research from the perspective of adsorption. Eng. Proc. 2022, 19, 20. [Google Scholar]
- Mohanakrishna, G.; Al-Raoush, R.I.; Abu-Reesh, I.M. Integrating electrochemical and bioelectrochemical systems for energetically sustainable treatment of produced water. Fuel 2021, 28, 119104. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of produced water in the Permian basin for hydraulic fracturing: Comparison of different coagulation processes and innovative filter media. Water 2020, 12, 770. [Google Scholar] [CrossRef]
- Huang, L.; Wu, B.; Wu, Y.; Yang, Z.; Yuan, T.; Alhassan, S.I.; Yang, W.; Wang, H.; Zhang, L. Porous and flexible membrane derived from ZIF-8-decorated hyphae for outstanding adsorption of Pb2+ ion. J. Colloid Interface Sci. 2020, 565, 465–473. [Google Scholar] [CrossRef]
- Kárászová, M.; Bourassi, M.; Gaálová, J. Membrane removal of emerging contaminants from water: Which kind of membranes should we use? Membranes 2020, 10, 305. [Google Scholar] [CrossRef]
- Goyal, P.; Tiwary, C.S.; Misra, S.K. Ion exchange based approach for rapid and selective Pb (II) removal using iron oxide dec-orated metal organic framework hybrid. J. Environ. Manag. 2020, 277, 111469. [Google Scholar] [CrossRef]
- Vaudevire, E.; Radmanesh, F.; Kolkman, A.; Vughs, D.; Cornelissen, E.; Post, J.; van der Meer, W. Fate and removal of trace pollutants from an anion exchange spent brine during the recovery process of natural organic matter and salts. Water Res. 2019, 154, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Hameed, B.H.; Almomani, F.A.; Usman, M.; Ba-Abbad, M.M.; Khraisheh, M. Dynamic simulation of lead(II) metal adsorption from water on activated carbons in a packed-bed column. Biomass Convers. Biorefin. 2022, in press. [Google Scholar] [CrossRef]
- Kuganathan, N.; Anurakavan, S.; Abiman, P.; Iyngaran, P.; Gkanas, E.I.; Chroneos, A. Adsorption of lead on the surfaces of pristine and B, Si and N-doped graphene. Phys. B Condens. Matter 2020, 600, 412639. [Google Scholar] [CrossRef]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy metal removal applications using adsorptive membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Okoro, H.K.; Pandey, S.; Ogunkunle, C.O.; Ngila, C.J.; Zvinowanda, C.; Jimoh, I.; Lawal, I.A.; Orosun, M.M.; Adeniyi, A.G. Nanomaterial-based biosorbents: Adsorbent for efficient removal of selected organic pollutants from industrial wastewater. Emerg. Contam. 2022, 8, 46–58. [Google Scholar] [CrossRef]
- Gök, G.; Kocyigit, H.; Gök, O.; Celebi, H. The use of raw shrimp shells in the adsorption of highly polluted waters with Co2+ Chem. Eng. Res. Des. 2022, 186, 229–240. [Google Scholar] [CrossRef]
- Kayranli, B.; Gok, O.; Yilmaz, T.; Gok, G.; Celebi, H.; Seckin, I.Y.; Mesutoglu, O.C. Low-cost organic adsorbent usage for removing Ni2+ and Pb2+ from aqueous solution and adsorption mechanisms. Int. J. Environ. Sci. Tech. 2022, 19, 3547–3564. [Google Scholar] [CrossRef]
- Tulun, Ş.; Akgül, G.; Alver, A.; Çelebi, H. Adaptive neuro-fuzzy interference system modelling for chlorpyrifos removal with walnut shell biochar. Arab. J. Chem. 2021, 14, 103443. [Google Scholar] [CrossRef]
- Sun, X.; Cui, B.; Yuan, B.; Wang, X.; Fan, L.; Yu, D.; He, Z.; Sheng, L.; Liu, J.; Lu, J. Liquid metal microparticles phase change medicated mechanical destruction for enhanced tumor cryoablation and dual-mode imaging. Adv. Funct. Mater. 2020, 30, 2003359. [Google Scholar] [CrossRef]
- Yun, G.; Tang, S.-Y.; Sun, S.; Yuan, D.; Zhao, Q.; Deng, L.; Yan, S.; Du, H.; Dickey, M.D.; Li, W. Liquid metal-filled magnetorheological elastomer with positive piezoconductivity. Nat. Commun. 2019, 10, 1300. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Tang, J.; Daeneke, T.; O’Mullane, A.P.; Stewart, L.A.; Liu, J.; Majidi, C.; Ruoff, R.S.; Weiss, P.S.; Dickey, M.D. Emergence of liquid metals in nanotechnology. ACS Nano 2019, 13, 7388–7395. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Rahim, M.A.; Tang, J. Low melting temperature liquid metals and their impacts on physical chemicstry. Acc. Mater. Res. 2021, 2, 8–577. [Google Scholar] [CrossRef]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, Y.; Liu, Z.; Wang, M. Liquid Metal-Based Devices: Material Properties, Fabrication and Functionalities. Nanomaterials 2021, 11, 3400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yao, S.; Rao, W.; Liu, J. Transformable soft liquid metal micro/nanomaterials. Mater. Sci. Eng. R Rep. 2019, 138, 1–35. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, V.; Sharma, P. Synthesis, characterization and evaluation of amphoteric galactomannan derivative for the mitigation of malachite green and congo red dye from aqueous solution. Cellulose 2022, 29, 1035–1053. [Google Scholar] [CrossRef]
- Noohi, Z.; Nosouhian, S.; Niroumand, B.; Timelli, G. Use of kow melting point metals and alloys (Tm < 420 °C) as phase change materials: A review. Metals 2022, 12, 945. [Google Scholar]
- Çelebi, H.; Gök, G.; Gök, O. Adsorption capability of brewed tea waste in waters containing toxic lead(II), cadmium (II), nickel (II), and zinc(II) heavy metal ions. Sci. Rep. 2020, 10, 17570. [Google Scholar] [CrossRef]
- Gök, G.; Çelebi, H. Laboratory scale elimination of some heavy metals with hollow aluminosilicate spheres. Int. J. Ecosys. Eco. Sci. 2019, 9, 305–312. [Google Scholar] [CrossRef]
- Çelebi, H.; Gök, O. Evaluation of lead adsorption kinetics and isotherms from aqueous solution using natural walnut shell. Int. J. Environ. Res. 2017, 11, 83–90. [Google Scholar] [CrossRef]

| Permissible Limits | |||
|---|---|---|---|
| WHO | USEPA | EPA | |
| 0.01 mg/L | 0.01 mg/L | 0.015 mg/L | |
| Properties | |||
| Density | Atomic Weight | Heat of fusion | Heat Capacity |
| 11.34 g/cm3 | 207.2 g/mol | 4.77 kJ/mol | 0.13 J/g K |
| Electron affinity | Boiling point | Melting Point | |
| 35.1 kJ/mol | 1740 °C | 327.5 °C | |
| Sources | |||
| Metal plating, Paint, Laundry process, Mining sector, Battery manufacturing, Steel industries, Alloys, Ceramics, Plastics, Glassware | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çelebi, H.; Bahadir, T.; Şimşek, İ.; Tulun, Ş. Reasons for High Adsorption Efficiencies in Lead Removal from Aquatic Solution. Eng. Proc. 2023, 31, 17. https://doi.org/10.3390/ASEC2022-13812
Çelebi H, Bahadir T, Şimşek İ, Tulun Ş. Reasons for High Adsorption Efficiencies in Lead Removal from Aquatic Solution. Engineering Proceedings. 2023; 31(1):17. https://doi.org/10.3390/ASEC2022-13812
Chicago/Turabian StyleÇelebi, Hakan, Tolga Bahadir, İsmail Şimşek, and Şevket Tulun. 2023. "Reasons for High Adsorption Efficiencies in Lead Removal from Aquatic Solution" Engineering Proceedings 31, no. 1: 17. https://doi.org/10.3390/ASEC2022-13812
APA StyleÇelebi, H., Bahadir, T., Şimşek, İ., & Tulun, Ş. (2023). Reasons for High Adsorption Efficiencies in Lead Removal from Aquatic Solution. Engineering Proceedings, 31(1), 17. https://doi.org/10.3390/ASEC2022-13812






