1. Introduction
Synthetic colorants, in particular tartrazine and brilliant blue FCF, are widely used in food chemistry and technology, although they can give negative health effects of various severities [
1,
2]. Both tartrazine and brilliant blue FCF are used as colorants for alcoholic and non-alcoholic beverages, candies, jellies, ice cream, etc. [
3]. The average daily intake of tartrazine and brilliant blue FCF is regulated at 7.5 mg/kg
bw [
4] and 6 mg/kg
bw [
5]. Therefore, colorant contents in foodstuffs have to be controlled.
Sensitive and selective, simple, and reliable methods for the quantification of these dyes are required. Voltammetric sensors suit well for such purposes. A wide range of electrochemical sensors has been developed for the determination of tartrazine. Brilliant blue FCF is a seldom studied colorant in electroanalytical chemistry. Most sensors are based on the application of surface modifiers, such as carbon nanomaterials [
6,
7,
8], metal [
9,
10] and metal oxide [
11] nanoparticles, polymeric coverages [
12,
13], and a combination of various modifiers [
14,
15,
16,
17,
18].
Tartrazine and brilliant blue FCF are often used together for the production of green-colored foodstuffs and beverages. Therefore, simultaneous voltammetric determination of these colorants is of practical interest. However, this topic did not receive enough attention. Just three voltammetric approaches have been reported [
19,
20,
21]. All of them are based on the application of modified electrodes (carbon black–polyethylene composite electrode [
19], ionic liquid-modified expanded graphite paste electrode [
20], and multi-walled carbon nanotube paste electrode [
21]).
Electrochemically inert metal oxide nanomaterials (TiO
2, In
2O
3, CeO
2, ZnO, Fe
3O
4, etc.) are perspective modifiers of the electrode surface [
22]. Their effectivity has been successfully demonstrated in the example of antioxidants [
23,
24,
25], pharmaceuticals [
26], and pollutants [
27,
28]. There is almost no application of such electrodes to food colorants excluding recent works focused on the tartrazine determination on CeO
2 [
29] and TiO
2 [
30,
31] nanoparticles-modified electrodes. Thus, the development of a voltammetric method based on the oxidation of tartrazine and brilliant blue FCF at the metal oxide nanomaterials-based electrodes is needed. In the current work, manganese dioxide nanorods (MnO
2 NR) dispersed in surfactant have been successfully applied as electrode surface modifiers. The electrode created provides well-resolved oxidation peaks of tartrazine and brilliant blue FCF allowing their simultaneous quantification.
2. Materials and Methods
Tartrazine (85% purity) was obtained from Sigma (St. Louis, MO, USA) and 85% brilliant blue FCF from Sigma-Aldrich (Steinheim, Germany). Ascorbic acid of 99% purity (Sigma, Steinheim, Germany), 9% sunset yellow and 99% vanillin (Aldrich, Steinheim, Germany), 99% sorbic acid, 98% riboflavin, and 85% indigo carmine (Sigma-Aldrich, Steinheim, Germany) were used for the interference study. In addition, 10 mM standard solutions of all compounds were prepared in distilled water. Other reagents were c.p. grade. The laboratory temperature was (25 ± 2 °C).
MnO2 NR (99%, diameter × L = 5–30 nm × 80–100 nm) from Sigma-Aldrich (Steinheim, Germany) were used as an electrode surface modifier. A 1 mg mL−1 suspension was prepared in 1.0 mM cetylpyridinium bromide using sonication for 40 min in an ultrasonic bath (WiseClean WUC-A03H (DAIHAN Scientific Co., Ltd., Wonju-si, Republic of Korea). A standard surfactant solution with a concentration of 1.0 mM was prepared from 98% cetylpyridinium bromide (Aldrich, Steinheim, Germany) by dissolving it in distilled water.
Voltammetric measurements were conducted on the potentiostat/galvanostat μAutolab Type III (Eco Chemie B.V., Utrecht, The Netherlands) and NOVA 1.7.8 software. Electrochemical impedance spectroscopy (EIS) was performed on the potentiostat/galvanostat Autolab PGSTAT 302N with the FRA 32M module (Eco Chemie B.V., Utrecht, The Netherlands) and the NOVA 1.10.1.9 software. A glassy electrochemical cell of 10 mL volume was used for electrochemical measurements. The tree-electrode system consisted of a working glassy carbon electrode (GCE) of 3 mm diameter (CH Instruments, Inc., Bee Cave, TX, USA), or a modified electrode, an Ag/AgCl reference electrode, and a platinum wire as an auxiliary electrode. After polishing on 0.05 µm alumina slurry, working electrode surface modification was performed by drop casting of 5 µL of MnO2 NR suspension.
The pH measurements were carried out using the “Expert-001” pH meter (Econix-Expert Ltd., Moscow, Russian Federation) with a glassy electrode.
A MerlinTM (Carl Zeiss, Oberkochen, Germany) high-resolution field emission scanning electron microscope was applied for the electrode surface morphology characterization and operated at a 5 kV accelerating voltage and a 300 pA emission current.
3. Results and Discussion
3.1. Voltammetric Characteristics of Colorants at Bare and Modified Electrodes
Tartrazine and brilliant blue FCF are electrochemically active on bare GCE in phosphate buffer pH 7.0. Single-step oxidation proceeds irreversibly which is typical for these colorants. The corresponding voltammetric characteristics are summarized in
Table 1.
Simultaneous detection of tartrazine and brilliant blue FCF at the bare GCE is impossible due to the full overlap of the oxidation peaks. Oxidation currents are low in spite of relatively high concentrations of colorants. Modifying with a MnO
2 NR electrode was used to solve this problem. The oxidation potential of colorants at the modified electrode is significantly changed (
Table 1). The difference in oxidation potential achieves 210 mV, making it possible to detect colorants simultaneously. There are two well-defined oxidation peaks on the voltammograms of the colorant’s mixture with a peak potential separation of 180 mV. Furthermore, oxidation currents at the modified electrode are statistically significantly increased which confirms the higher sensitivity of the colorant’s response and the effectivity of the suggested modifier.
3.2. Morphology, Effective Surface Area, and Electron Transfer Properties of the Modified Electrode
Scanning electron microscopy data confirm the presence of a modifier on the GCE surface (
Figure 1). A sponge-like structure from the intertwined nanorods with a width of 15–20 nm included in the surfactant film has been obtained for MnO
2 NR (
Figure 1b).
Electrochemical investigation of redox peaks of 1.0 mM [Fe(CN)6]4− ions has shown that the modified electrode demonstrates a significant increase in the effective surface area compared to bare GCE (70 ± 2 mm2 vs. 8.9 ± 0.3 mm2 for bare GCE). This explains the increase in the colorant’s oxidation currents at the modified electrode.
EIS in the presence of 1.0 mM [Fe(CN)6]4−/3− as a redox probe was used for the characterization of the electron transfer properties of the electrodes. The 72-fold decrease (72 ± 3 kOhm vs. 1.0 ± 0.2 kOhm for GCE) of the charge transfer resistance clearly confirms the increase in the electron transfer rate at the modified electrode. The constant phase element was increased 29-fold compared to bare GCE which is caused by the porous structure of the modified electrode surface as well as by the increase in the total surface charge due to the presence of a cationic surfactant.
The data obtained confirm once more the effectivity of MnO2 NR as an electrode surface modifier.
3.3. Simultaneous Determination of Tartrazine and Brilliant Blue FCF
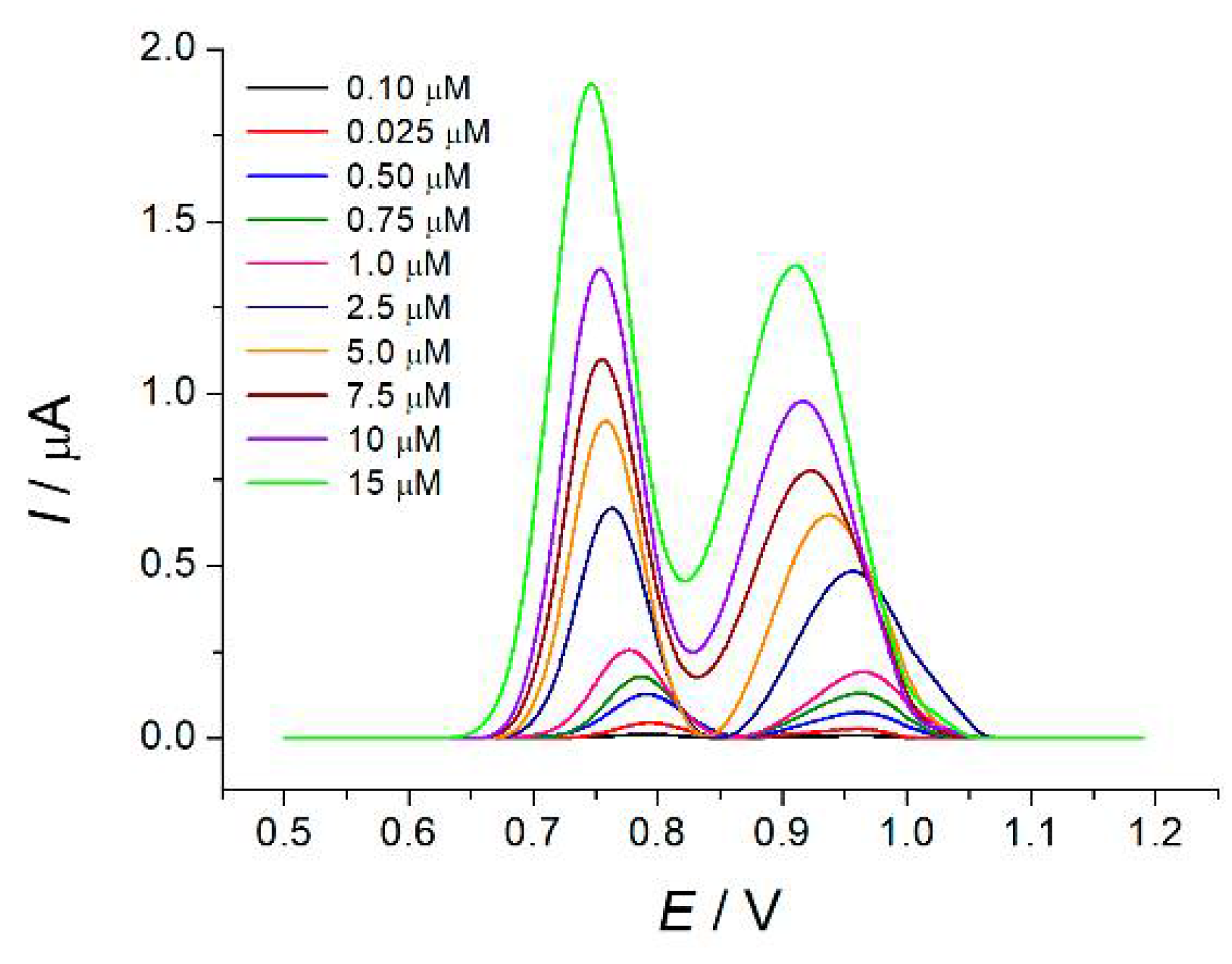
Differential pulse voltammetry was used for the simultaneous quantification of tartrazine and brilliant blue FCF in phosphate buffer pH 7.0. Well-resolved oxidation peaks of colorants at 0.77 and 0.97 V for tartrazine and brilliant blue FCF, respectively, were observed on the voltammograms (
Figure 2). Oxidation currents increase linearly with the growth of the colorant’s concentration in the ranges of 0.10–2.5 and 2.5–15 µM for tartrazine and 0.25–2.5 and 2.5–15 µM for brilliant blue FCF with detection limits of 0.043 and 0.041 µM, respectively. The limits of detection are worse than for the other electrodes for the determination of tartrazine and brilliant blue FCF [
19,
20] but improved vs. multi-walled carbon nanotubes–carbon paste electrode [
21]. Nevertheless, simultaneous determination is impossible at the carbon ink film-modified carbon black–polyethylene composite electrode [
19] as far as detection is performed at the various pH for tartrazine and brilliant blue FCF. Method [
20] requires pre-concentration for 500 s complicating the measurement procedure.
Voltammograms for the non-equimolar mixtures of colorants indicate their independent oxidation in the first linear range. Therefore, calibration graphs obtained for equimolar mixtures are universal and can be used independently of the colorant’s concentration ratio in the sample. Simple dilution can be applied in the case of high contents of the colorants in real samples.
The accuracy of the method developed has been shown on the model mixtures of colorants at five concentration levels. The relative standard deviation of the determination does not exceed 3% confirming the high reproducibility of the electrode response (the electrode was renewed before each measurement). The recovery value is in the range of 99–100% confirming the high accuracy of the sensor developed.
Foodstuffs are characterized by a multi-component composition which can affect the response of colorants. The selectivity test has shown that typical interferences (inorganic ions (1000-fold excess of K
+, Mg
2+, Ca
2+, NO
3−, Cl
−, and SO
42−), saccharides (100-fold excess of glucose, rhamnose, and sucrose), 10-fold excess of ascorbic acid, and electrochemically silent sorbic acid), equimolar level of vanillin, and other food colorants (50-fold excess of riboflavin, 10-fold excess of indigo carmine, and equimolar level of sunset yellow) do not affect the response of tartrazine and brilliant blue FCF. Thus, the high selectivity of the electrode created towards tartrazine and brilliant blue FCF is an important advantage over other electrodes [
19,
20,
21].
Practical application of the electrode has been demonstrated on soft and isotonic sports drinks. Sample 1 is free of tartrazine while samples 2–4 contain both colorants but the concentration of brilliant blue FCF is too low and cannot be determined by voltammetry. Standard addition method data confirm that oxidation peaks of real samples belong to the colorants. The results of soft and isotonic sports drinks analysis are presented in
Figure 3. Voltammetric data agree well with that obtained by high-performance liquid chromatography [
32].
t- and
F-tests confirm the absence of systematic errors of determination and similar precision of both methods.
4. Conclusions
An electrode modified with MnO2 NR was developed for the determination of tartrazine and brilliant blue FCF for the first time. The simultaneous determination of colorants in the ranges of 0.10–2.5 and 2.5–15 µM of tartrazine and 0.25–2.5 and 2.5–15 µM of brilliant blue FCF was achieved using the electrode created. The high selectivity of the electrode response to target colorants is a major advantage of the approach developed. The voltammetric method developed is simple, highly selective, express, and reliable and can be used for beverage quality control.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z.; validation, L.G. and G.Z.; investigation, L.G.; writing—original draft preparation, G.Z.; writing—review and editing, G.Z.; visualization, L.G.; supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank Aleksei Rogov (Laboratory of Scanning Electron Microscopy, Interdisciplinary Center for Analytical Microscopy, Kazan Federal University) for the scanning electron microscopy measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A.; Nurudeen, J.; Orovwigho, U.; Ahiabuike, J. Assessment of the health implications of synthetic and natural food colourants. UK J. Pharm. Biosci. 2016, 4, 1–11. [Google Scholar]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food Colour Additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef]
- Scientific Opinion. On re-evaluation of Tartrazine (E102) as a food additive. EFSA J. 2009, 7, 1331. [Google Scholar] [CrossRef]
- Scientific Opinion. On the re-evaluation of brilliant blue FCF (E133) as a food additive. EFSA J. 2010, 8, 1853. [Google Scholar] [CrossRef]
- Sierra-Rosales, P.; Toledo-Neira, C.; Squella, J.A. Electrochemical determination of food colorants in soft drinks using MWCNT-modified GCEs. Sens. Actuat. B 2017, 240, 1257–1264. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Duangmal, K.; Chailapakul, O. Electrochemically reduced graphene oxide-modified screen-printed carbon electrodes for a simple and highly sensitive electrochemical detection of synthetic colorants in beverages. Talanta 2016, 160, 113–124. [Google Scholar] [CrossRef]
- Sierra-Rosales, P.; Toledo-Neira, C.; Ortúzar-Salazar, P.; Squella, J.A. MWCNT-modified electrode for voltammetric determination of allura red and brilliant blue FCF in isotonic sport drinks. Electroanalysis 2019, 31, 883–890. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Aleshi, M. Electrochemical modification of glassy-carbon electrode by bismuth-chitosan nanosheets for electrocatalytic reduction and determination of tartrazine. Portugal. Electrochim. Acta 2014, 32, 369–379. [Google Scholar] [CrossRef]
- Kolozof, P.-A.; Florou, A.B.; Spyrou, K.; Hrbac, J.; Prodromidis, M.I. In-situ tailoring of the electrocatalytic properties of screen-printed graphite electrodes with sparked generated molybdenum nanoparticles for the simultaneous voltammetric determination of sunset yellow and tartrazine. Sens. Actuat. B 2020, 304, 127268. [Google Scholar] [CrossRef]
- Marquez-Mariño, K.; Penagos-Llanos, J.; García-Beltrán, O.; Nagles, E.; Hurtado, J.J. Development of a novel electrochemical sensor based on a carbon paste electrodedecorated with Nd2O3 for the simultaneous detection of tartrazine and sunset yellow. Electroanalysis 2018, 30, 2760–2767. [Google Scholar] [CrossRef]
- Sun, S.-C.; Hsien, B.-C.; Chuang, M.-C. Electropolymerised-hemin-catalysed reduction and analysis of tartrazine and sunset yellow. Electrochim. Acta 2019, 319, 766–774. [Google Scholar] [CrossRef]
- Chao, M.; Ma, X. Convenient electrochemical determination of sunset yellow and tartrazine in food samples using a poly(L-phenylalanine)-modified glassy carbon electrode. Food Anal. Methods 2015, 8, 130–138. [Google Scholar] [CrossRef]
- Tahtaisleyen, S.; Gorduk, O.; Sahin, Y. Electrochemical determination of tartrazine using a graphene/poly(L-phenylalanine) modified pencil graphite electrode. Anal. Lett. 2020, 53, 1683–1703. [Google Scholar] [CrossRef]
- Yu, L.; Zheng, H.; Shi, M.; Jing, S.; Qu, L. A novel electrochemical sensor based on poly(diallyldimethylammonium chloride)-dispersed graphene supported palladium nanoparticles for simultaneous determination of sunset yellow and tartrazine in soft drinks. Food Anal. Methods 2017, 10, 200–209. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Khorablou, Z.; Zamani, M.; Dorraji, P.S.; Alamgholiloo, M. Voltammetric sensor for tartrazine determination in soft drinks using poly (p-aminobenzenesulfonic acid)/zinc oxide nanoparticles in carbon paste electrode. J. Food Drug Anal. 2017, 25, 293–301. [Google Scholar] [CrossRef]
- Nuñez-Dallos, N.; Macías, M.A.; García-Beltrán, O.; Calderón, J.A.; Nagles, E.; Hurtado, J. Voltammetric determination of amaranth and tartrazine with a new double-stranded copper(I) helicate-single-walled carbon nanotube modified screen printed electrode. J. Electroanal. Chem. 2018, 822, 95–104. [Google Scholar] [CrossRef]
- Wang, M.; Yang, M.; Suin, Q.; Cao, Y.; Zhao, J. Development of a facile sensor for the determination of brilliant blue FCF in beverages. Int. J. Environ. Anal. Chem. 2015, 95, 969–979. [Google Scholar]
- Lipskikh, O.I.; Korotkova, E.I.; Barek, J.; Vyskocil, V.; Saqib, M.; Khristunova, E.P. Simultaneous voltammetric determination of Brilliant Blue FCF and Tartrazine for food quality control. Talanta 2020, 218, 121136. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Zhang, J.; Wang, X.; Chen, Z. Electrochemical determination of brilliant blue and tartrazine based on an ionic liquid-modified expanded graphite paste electrode. J. AOAC Int. 2015, 98, 817–821. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous voltammetric determination of Brilliant Blue and Tartrazine in real samples at the surface of a multi-walled carbon nanotube paste electrode. Anal. Methods 2011, 3, 2842–2847. [Google Scholar] [CrossRef]
- Agnihotri, A.S.; Varghese, A.; Nidhin, N. Transition metal oxides in electrochemical and bio sensing: A state-of-art review. Appl. Surf. Sci. Adv. 2021, 4, 100072. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Gimadutdinova, L. Cerium(IV) and iron(III) oxides nanoparticles based voltammetric sensor for the sensitive and selective determination of lipoic acid. Sensors 2021, 21, 7639. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Yakupova, E.; Davletshin, R. Voltammetric determination of hesperidin on the electrode modified with SnO2 nanoparticles and surfactants. Electroanalysis 2021, 33, 2417–2427. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Romashkina, S.; Budnikov, H. Highly sensitive amperometric sensor for eugenol quantification based on CeO2 nanoparticles and surfactants. Electroanalysis 2017, 29, 1197–1204. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Erkmen, C.; Kurbanoglu, S.; Ozkan, S.A.; Uslu, B. Electrochemical analysis for pharmaceuticals by the advantages of metal oxide nanomaterials. Curr. Anal. Chem. 2021, 17, 1322–1339. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Yuan, C.; Zhang, F.; Ma, L.; Qin, D.; Shan, D.; Lu, X. A novel electrochemical sensor based on zirconia/ordered macroporous polyaniline for ultrasensitive detection of pesticides. Analyst 2015, 140, 560–566. [Google Scholar] [CrossRef]
- Zaidi, S.A.; Shin, J.H. A novel and highly sensitive electrochemical monitoring platform for 4-nitrophenol on MnO2 nanoparticles modified graphene surface. RSC Adv. 2015, 5, 88996–89002. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Voltammetric determination of tartrazine on an electrode modified with cerium dioxide nanoparticles and cetyltriphenylphosphonium bromide. J. Anal. Chem. 2022, 77, 664–670. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and Selective Detection of Tartrazine Based on TiO2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.; Liu, Y.; Wu, J.; Li, G.; Liu, J.; He, Q. A simple but efficient voltammetric sensor for simultaneous detection of tartrazine and ponceau 4R based on TiO2/electro-reduced graphene oxide nanocomposite. Chemosensors 2020, 8, 70. [Google Scholar] [CrossRef]
- Harp, B.P.; Miranda-Bermudez, E.; Barrows, J.N. Determination of seven certified color additives in food products using liquid chromatography. J. Agric. Food Chem. 2013, 61, 3726–3736. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).