Highly Sensitive Determination of Hesperidin Using Electrode Modified with Poly(Ferulic Acid) †
Abstract
:1. Introduction
2. Materials and Methods
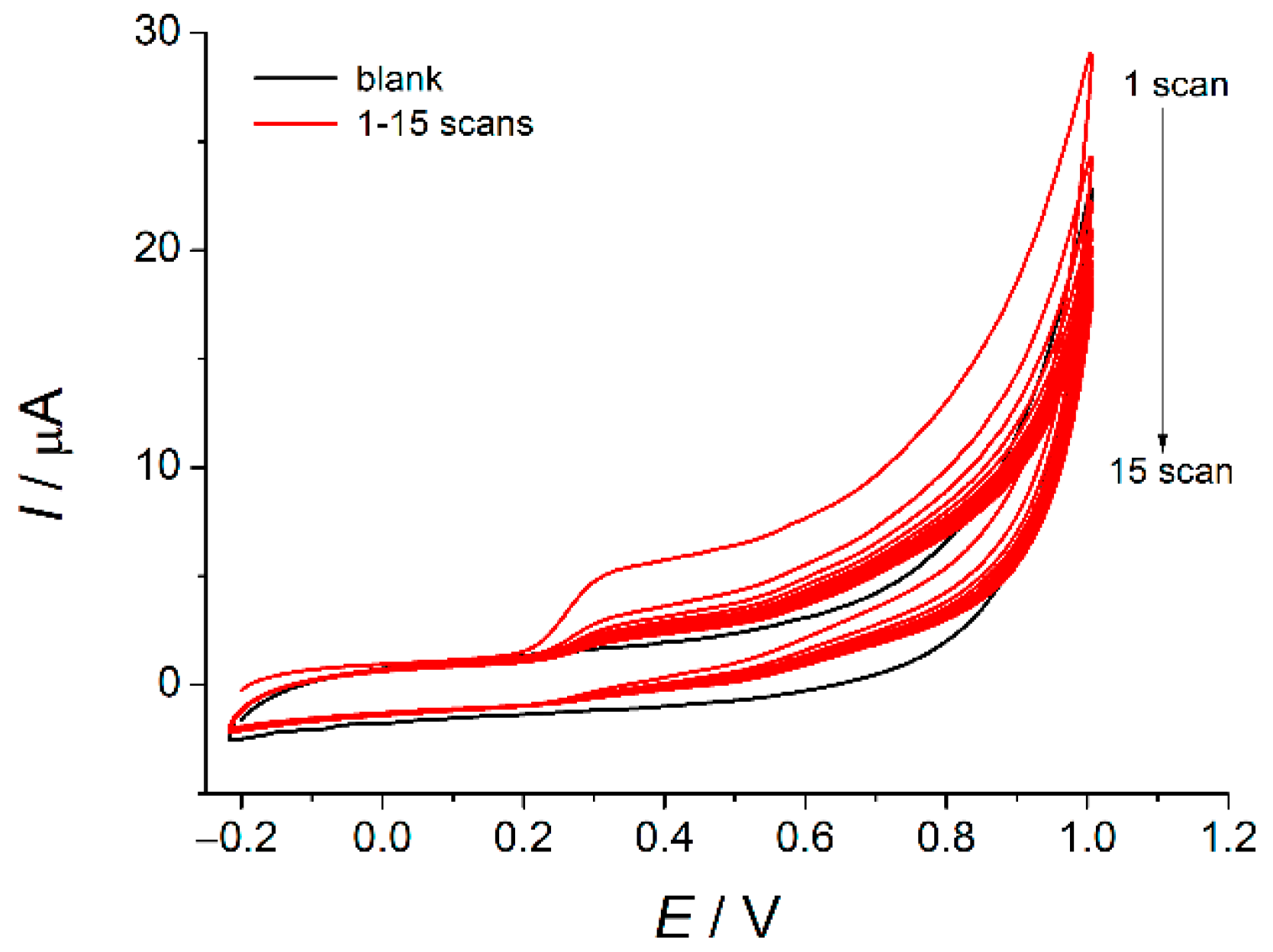
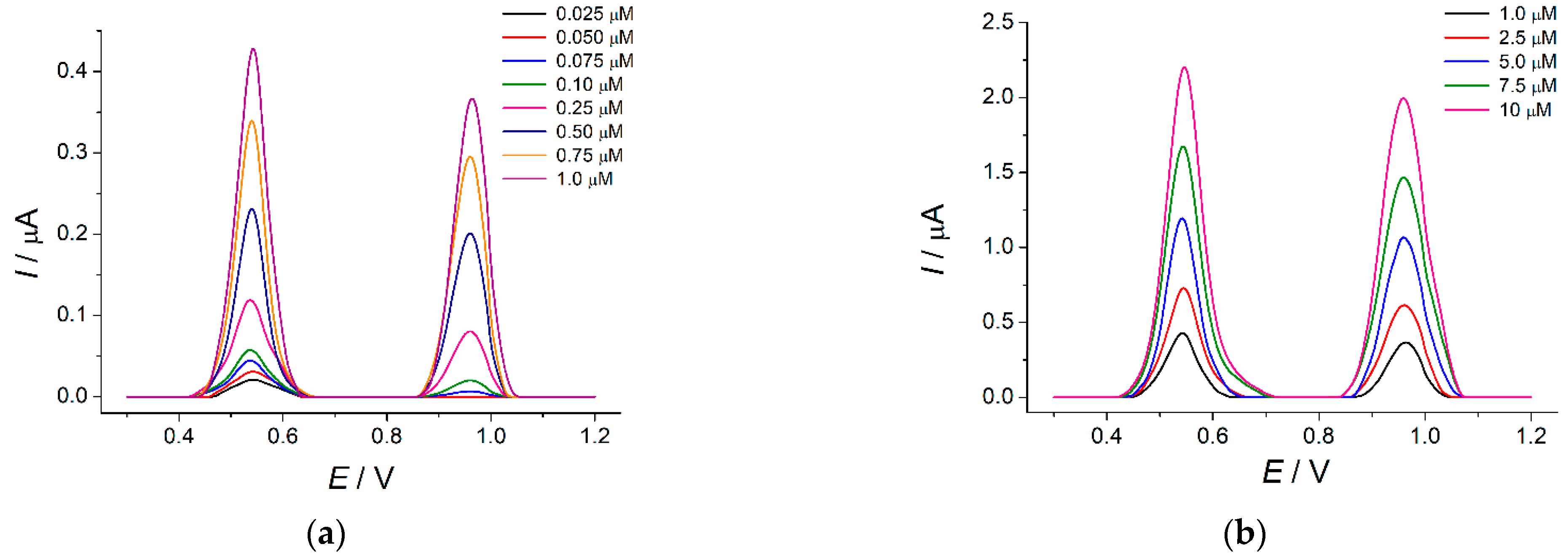
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of hesperidin on cardiovascular disease risk factors: The role of intestinal microbiota on hesperidin bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Pla-Pagà, L.; Companys, J.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: A randomized controlled trial (Citrus study). Eur. J. Nutr. 2021, 60, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Yiğit, A.; Yardım, Y.; Şentürk, Z. Square-wave adsorptive stripping voltammetric determination of hesperidin using a boron-doped diamond electrode. J. Anal. Chem. 2020, 75, 653–661. [Google Scholar] [CrossRef]
- David, I.G.; Numan, N.; Buleandră, M.; Popa, D.-E.; Lițescu, S.C.; Riga, S.; Ciobanu, A.M. Rapid voltammetric screening method for the assessment of bioflavonoid content using the disposable bare pencil graphite electrode. Chemosensors 2021, 9, 323. [Google Scholar] [CrossRef]
- Šafranko, S.; Stanković, A.; Asserghine, A.; Jakovljević, M.; Hajra, S.; Nundy, S.; Medvidović-Kosanović, M.; Jokić, S. Electroactivated disposable pencil graphite electrode—New, cost-effective, and sensitive electrochemical detection of bioflavonoid hesperidin. Elecrtoanalysis 2021, 33, 1063–1071. [Google Scholar] [CrossRef]
- Sims, M.J.; Li, Q.; Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Using multiwalled carbon nanotube modified electrodes for the adsorptive striping voltammetric determination of hesperidin. Electrochim. Acta 2009, 54, 5030–5034. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, L.; Chen, G. Carbon nanotube-phenolic resin composite electrode fabricated by far infrared-assisted crosslinking for enhanced amperometric detection. Electroanalysis 2019, 31, 756–765. [Google Scholar] [CrossRef]
- Xia, H.-q.; Gu, T.; Fan, R.; Zeng, J. Comparative investigation of bioflavonoid electrocatalysis in 1D, 2D, and 3D carbon nanomaterials for simultaneous detection of naringin and hesperidin in fruits. RSC Adv. 2022, 12, 6409–6415. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Yakupova, E.; Davletshin, R. Voltammetric determination of hesperidin on the electrode modified with SnO2 nanoparticles and surfactants. Electroanalysis 2021, 33, 2417–2427. [Google Scholar] [CrossRef]
- Sun, D.; Wang, F.; Wu, K.; Chen, J.; Zhou, Y. Electrochemical determination of hesperidin using mesoporous SiO2 modified electrode. Microchim. Acta 2009, 167, 35–39. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Wang, H.; Lu, W.; Guo, M. Highly sensitive detection of hesperidin using AuNPs/rGO modified glassy carbon electrode. Analyst 2018, 143, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Zhupanova, A.; Guss, E.; Ziyatdinova, G.; Budnikov, H. Simultaneous voltammetric determination of flavanones using an electrode based on functionalized single-walled carbon nanotubes and polyaluminon. Anal. Lett. 2020, 53, 2170–2189. [Google Scholar] [CrossRef]
- Sun, B.; Hou, X.; Li, D.; Gou, Y.; Hu, F.; Li, W.; Shi, X. Electrochemical sensing and high selective detection of hesperidin with molecularly imprinted polymer based on ultrafine activated carbon. J. Electrochem. Soc. 2019, 166, B1644–B1652. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical Sensors Based on the Electropolymerized Natural Phenolic Antioxidants and Their Analytical Application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Yakupova, E.; Guss, E.; Budnikov, H. The selective electrochemical sensing of naringin using electropolymerized ellagic acid film. J. Electrochem. Soc. 2020, 167, 107502. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Budnikov, H. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
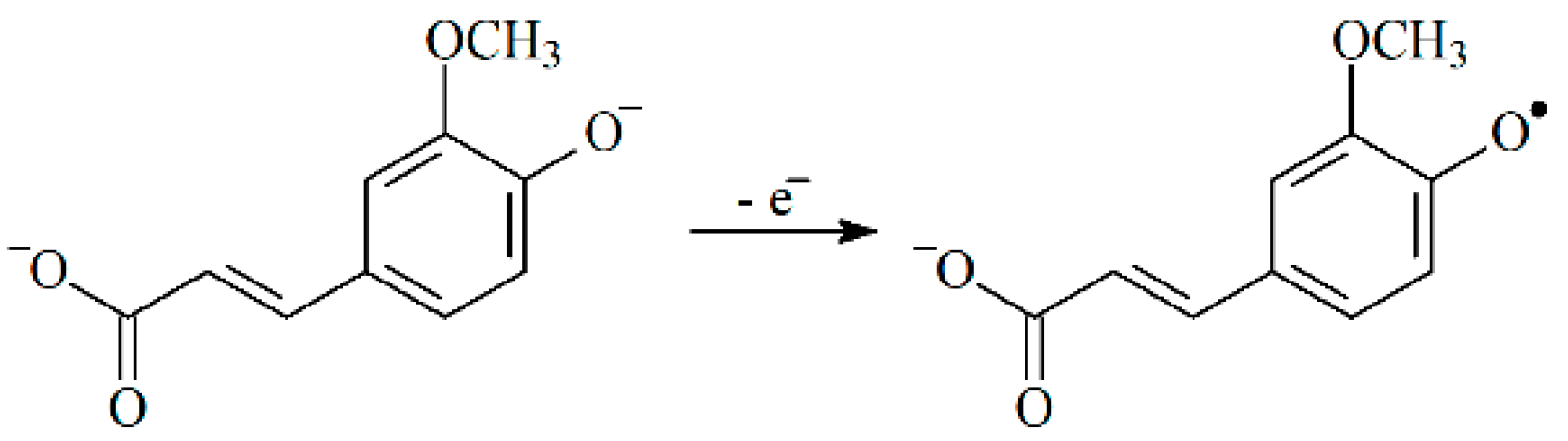
| Electrode | Eox1 (V) | Iox1 (μA) | Eox2 (V) | Iox2 (μA) |
|---|---|---|---|---|
| GCE | 0.563 | 0.056 ± 0.002 | 0.935 | 0.045 ± 0.002 |
| MWCNTs/GCE | 0.504 | 0.18 ± 0.01 | 0.886 | 0.23 ± 0.01 |
| Poly (ferulic acid)/MWCNTs/GCE | 0.509 | 0.50 ± 0.02 | 0.904 | 0.42 ± 0.02 |
| Added Amount (µg) | Found Amount (µg) | RSD (%) | R (%) |
|---|---|---|---|
| 0.0610 | 0.061 ± 0.002 | 3.2 | 100 |
| 0.610 | 0.61 ± 0.01 | 1.6 | 100 |
| 2.44 | 2.43 ± 0.04 | 1.4 | 99.6 |
| 12.2 | 12.2 ± 0.1 | 0.79 | 100 |
| 24.4 | 24.3 ± 0.3 | 1.0 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakupova, E.; Ziyatdinova, G. Highly Sensitive Determination of Hesperidin Using Electrode Modified with Poly(Ferulic Acid). Eng. Proc. 2023, 31, 1. https://doi.org/10.3390/ASEC2022-13786
Yakupova E, Ziyatdinova G. Highly Sensitive Determination of Hesperidin Using Electrode Modified with Poly(Ferulic Acid). Engineering Proceedings. 2023; 31(1):1. https://doi.org/10.3390/ASEC2022-13786
Chicago/Turabian StyleYakupova, Elvira, and Guzel Ziyatdinova. 2023. "Highly Sensitive Determination of Hesperidin Using Electrode Modified with Poly(Ferulic Acid)" Engineering Proceedings 31, no. 1: 1. https://doi.org/10.3390/ASEC2022-13786
APA StyleYakupova, E., & Ziyatdinova, G. (2023). Highly Sensitive Determination of Hesperidin Using Electrode Modified with Poly(Ferulic Acid). Engineering Proceedings, 31(1), 1. https://doi.org/10.3390/ASEC2022-13786







