Curing Characteristics of Urethane-Dimethacrylate Homopolymers and Their Composites for Potential Application in Bone Cement †
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Curing Procedure
2.3. Curing Temperature and Time
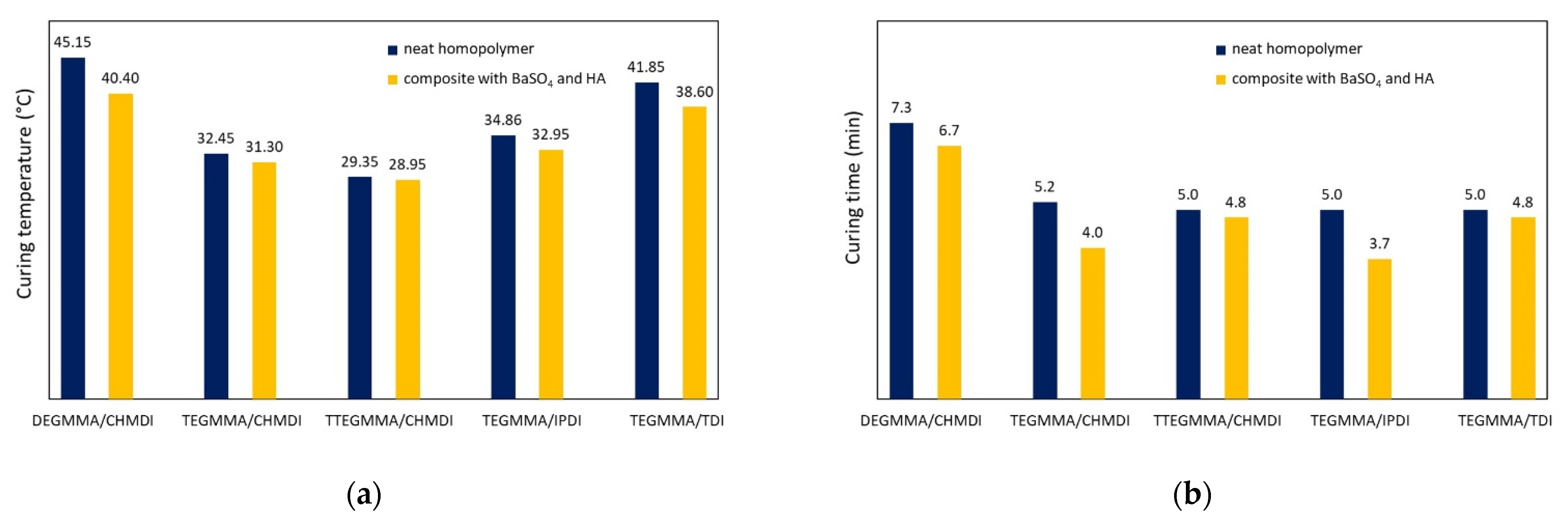
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone Cement. J. Clin. Orthop. Trauma 2013, 4, 157. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Albanese, C.; Vernè, E.; Miola, M. PMMA-Based Bone Cements and the Problem of Joint Arthroplasty Infections: Status and New Perspectives. Materials 2019, 12, 4002. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhang, B.; Yan, L. A Preliminary Review of Modified Polymethyl Methacrylate and Calcium-Based Bone Cement for Improving Properties in Osteoporotic Vertebral Compression Fractures. Front. Mater. 2022, 9, 9127131. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Hassanzadeh Tabrizi, S.A.; Ismail, A.F.; Seifalian, A.; RamaKrishna, S.; Berto, F. Poly(Methyl Methacrylate) Bone Cement, Its Rise, Growth, Downfall and Future. Polym. Int. 2021, 70, 1182–1201. [Google Scholar] [CrossRef]
- DiPisa, J.A.; Sih, G.C.; Berman, A.T. The Temperature Problem at the Bone-Acrylic Cement Interface of the Total Hip Replacement. Clin. Orthop. Relat. Res. 1976, 121, 95–98. [Google Scholar] [CrossRef]
- ISO 5833:2002; Implants for Surgery—Acrylic Resin Cements. International Standard Organisation: London, UK, 2002.
- Gundapaneni, D.; Goswami, T. Thermal Isotherms in PMMA and Cell Necrosis during Total Hip Arthroplasty. J. Appl. Biomater. Funct. Mater. 2014, 12, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The Adverse Health Effects of Bisphenol A and Related Toxicity Mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.M. Characterization of Urethane-Dimethacrylate Derivatives as Alternative Monomers for the Restorative Composite Matrix. Dent. Mater. 2014, 30, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.; Gibas, M.; Kurcok, M. Evaluation of the Network Parameter in Aliphatic Poly(Urethane Dimethacrylate)s by Dynamic Thermal Analysis. Polymer 2000, 41, 3129–3135. [Google Scholar] [CrossRef]
- Liptáková, T.; Lelovics, H.; Nečas, L. Variations of Temperature of Acrylic Bone Cements Prepared by Hand and Vacuum Mixing during Their Polymerization. Acta Bioeng. Biomech. 2009, 11, 47–51. [Google Scholar] [PubMed]
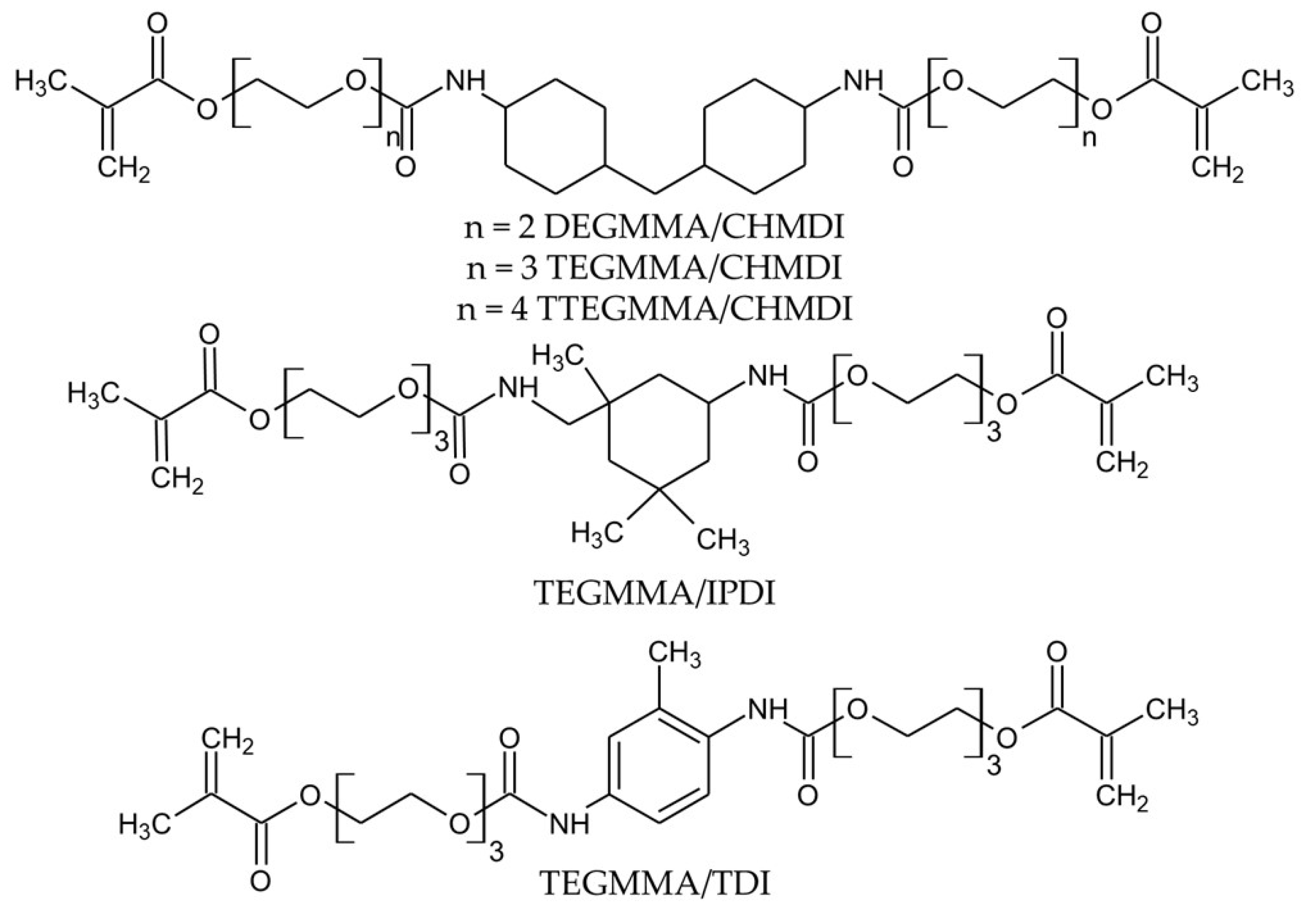

| Monomer | BPO (wt.%) | DMPT (wt.%) |
|---|---|---|
| DEGMMA/CHMDI | 0.5 | 0.15 |
| TEGMMA/CHMDI | 0.6 | 0.15 |
| TTEGMMA/CHMDI | 0.6 | 0.15 |
| TEGMMA/IPDI | 1.1 | 0.2 |
| TEGMMA/TDI | 0.4 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrószcz-Porębska, M.W.; Barszczewska-Rybarek, I.M. Curing Characteristics of Urethane-Dimethacrylate Homopolymers and Their Composites for Potential Application in Bone Cement. Eng. Proc. 2023, 31, 11. https://doi.org/10.3390/ASEC2022-13798
Chrószcz-Porębska MW, Barszczewska-Rybarek IM. Curing Characteristics of Urethane-Dimethacrylate Homopolymers and Their Composites for Potential Application in Bone Cement. Engineering Proceedings. 2023; 31(1):11. https://doi.org/10.3390/ASEC2022-13798
Chicago/Turabian StyleChrószcz-Porębska, Marta W., and Izabela M. Barszczewska-Rybarek. 2023. "Curing Characteristics of Urethane-Dimethacrylate Homopolymers and Their Composites for Potential Application in Bone Cement" Engineering Proceedings 31, no. 1: 11. https://doi.org/10.3390/ASEC2022-13798
APA StyleChrószcz-Porębska, M. W., & Barszczewska-Rybarek, I. M. (2023). Curing Characteristics of Urethane-Dimethacrylate Homopolymers and Their Composites for Potential Application in Bone Cement. Engineering Proceedings, 31(1), 11. https://doi.org/10.3390/ASEC2022-13798







