A Sulfo-Cyanine Dye as a Colorimetric Chemosensor for Metal Cation Recognition †
Abstract
1. Introduction
2. Experimental Section
2.1. Methods and Materials
2.2. Photophysical Characterization
2.3. Preliminary Chemosensing Studies and Spectrophotometric Titrations
3. Results and Discussion
3.1. Photophysical Characterization
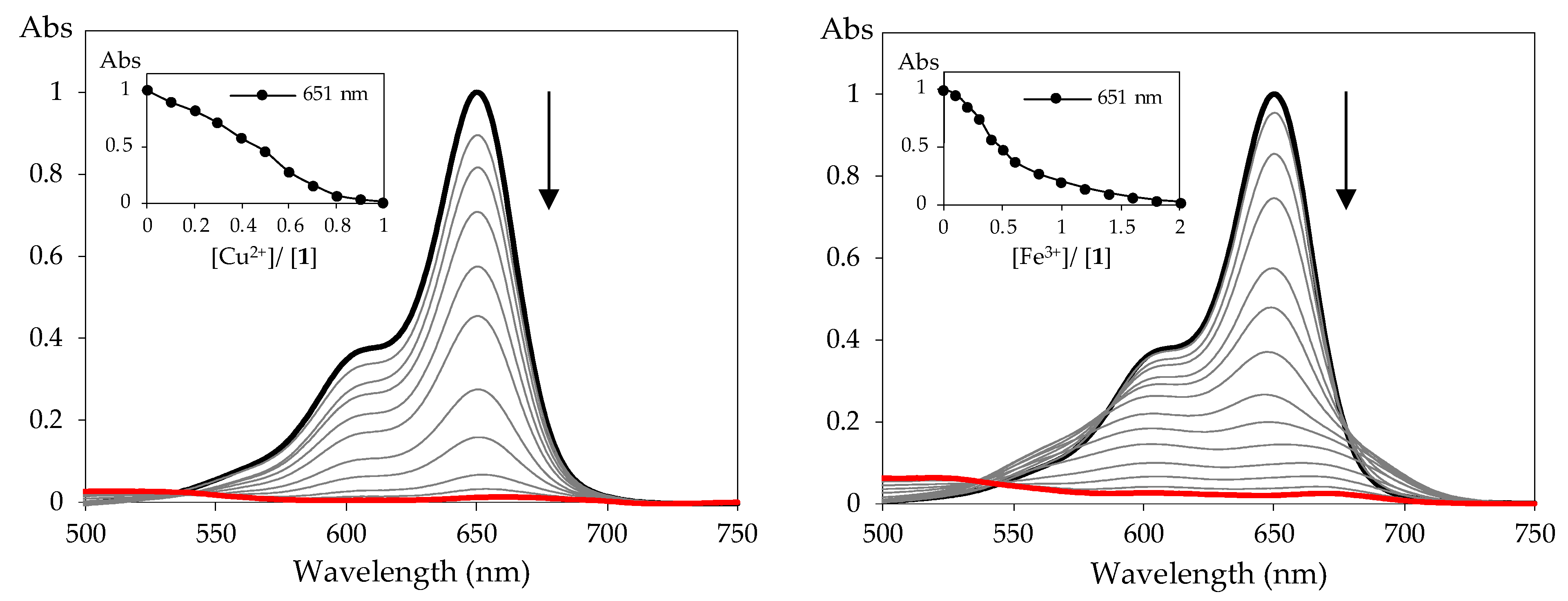
3.2. Preliminary Chemosensing Studies and Spectrophotometric Titrations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- You, L.; Zha, D.; Anslyn, E.V. Recent advances in supramolecular analytical chemistry using optical sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors—A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Mohammadi, A.; Yaghoubi, S. A new dual colorimetric chemosensor based on quinazolinone for CN−, AcO− and Cu2+ ions. Sens. Actuators B Chem. 2017, 241, 1069–1075. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, K.; Li, J.-Y.; Fang, Y.; Zhao, T.-C.; Yao, C. Visualization of nitroxyl in living cells by a chelated copper(II) coumarin complex. Org. Lett. 2011, 13, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Gai, L.; Chen, H.; Zou, B.; Lu, H.; Lai, G.; Li, Z.; Shen, Z. Ratiometric fluorescence chemodosimeters for fluoride anion based on pyrene excimer/monomer transformation. Chem. Commun. 2012, 48, 10721–10723. [Google Scholar] [CrossRef]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent chemosensors based on spiro ring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef]
- Presti, M.L.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M.; Sancenón, F. A dual channel sulphur-containing macrocycle functionalised BODIPY probe for the detection of Hg(II) in mixed aqueous solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Wolf, N.; Kersting, L.; Herok, C.; Mihm, C.; Seibel, J. High-yielding water-soluble asymmetric cyanine dyes for labeling applications. J. Org. Chem. 2020, 85, 9751–9760. [Google Scholar] [CrossRef]
- Owens, E.A.; Bruschi, N.; Tawney, J.G.; Henary, M. A microwave-assisted and environmentally benign approach to the synthesis of near-infrared fluorescent pentamethine cyanine dyes. Dyes Pigm. 2015, 113, 27–37. [Google Scholar] [CrossRef]
- Yi, X.; Wang, F.; Qin, W.; Yang, X.; Yuan, J. Near-infrared fluorescent probes in cancer imaging and therapy: An emerging field. Int. J. Nanomed. 2014, 9, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yin, J.; Yoon, J. A multi-responsive cyanine-based colorimetric chemosensor containing dipicolylamine moieties for the detection of Zn(II) and Cu(II) ions. Sens. Actuators B Chem. 2016, 230, 40–45. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, R.; Yuan, W.; Liu, Q.; Shi, M.; Feng, W.; Wu, Z.; Hu, K.; Li, F. An easy-to-use colorimetric cyanine probe for the detection of Cu2+ in Wilson’s Disease. ACS Appl. Mater. Interfaces 2018, 10, 20377–20386. [Google Scholar] [CrossRef]
- Martins, C.D.F.; Batista, P.M.R.; Raposo, M.M.M.; Costa, S.P.G. Crown ether benzoxazolyl-alanines as fluorimetric chemosensors for the detection of palladium in aqueous environment. Chem. Proc. 2021, 3, 5. [Google Scholar]
- Esteves, C.I.C.; Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. New fluoroionophores for metal cations based on benzo[d]oxazol-5-yl-alanine bearing pyrrole and imidazole. Dyes Pigm. 2018, 151, 211–218. [Google Scholar] [CrossRef]
- Okda, H.E.; El Sayed, S.; Ferreira, R.C.M.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. N,N-diphenylanilino-heterocyclic aldehyde-based chemosensors for UV-vis/NIR and fluorescence Cu(II) detection. New J. Chem. 2019, 43, 7393–7402. [Google Scholar] [CrossRef]
- Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. Synthesis and characterization of a water-soluble pentamethine indocyanine dye for peptide labeling. Chem. Proc. 2022, 8, 91. [Google Scholar]
- Jose, J.; Ueno, Y.; Burgess, K. Water-soluble Nile Blue derivatives: Syntheses and photophysical properties. Chem. Eur. J. 2009, 15, 418–423. [Google Scholar] [CrossRef]
- Wang, L.; Fan, J.; Qiao, X.; Peng, X.; Dai, B.; Wang, B.; Sun, S.; Zhang, L.; Zhang, Y. Novel asymmetric Cy5 dyes: Synthesis, photostabilities and high sensitivity in protein fluorescence labeling. J. Photochem. Photobiol. A Chem. 2010, 210, 168–172. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Lee, W.; Ko, G.; Yin, J.; Yoon, J. Recent progress in the development of organic dye based near-infrared fluorescence probes for metal ions. Coord. Chem. Rev. 2018, 354, 74–97. [Google Scholar] [CrossRef]
- Li, S.; Zhang, D.; Xie, X.; Ma, S.; Liu, Y.; Xu, Z.; Gao, Y.; Ye, Y. A novel solvent-dependently bifunctional NIR absorptive and fluorescent ratiometric probe for detecting Fe3+/Cu2+ and its application in bioimaging. Sens. Actuators B Chem. 2016, 224, 661–667. [Google Scholar] [CrossRef]
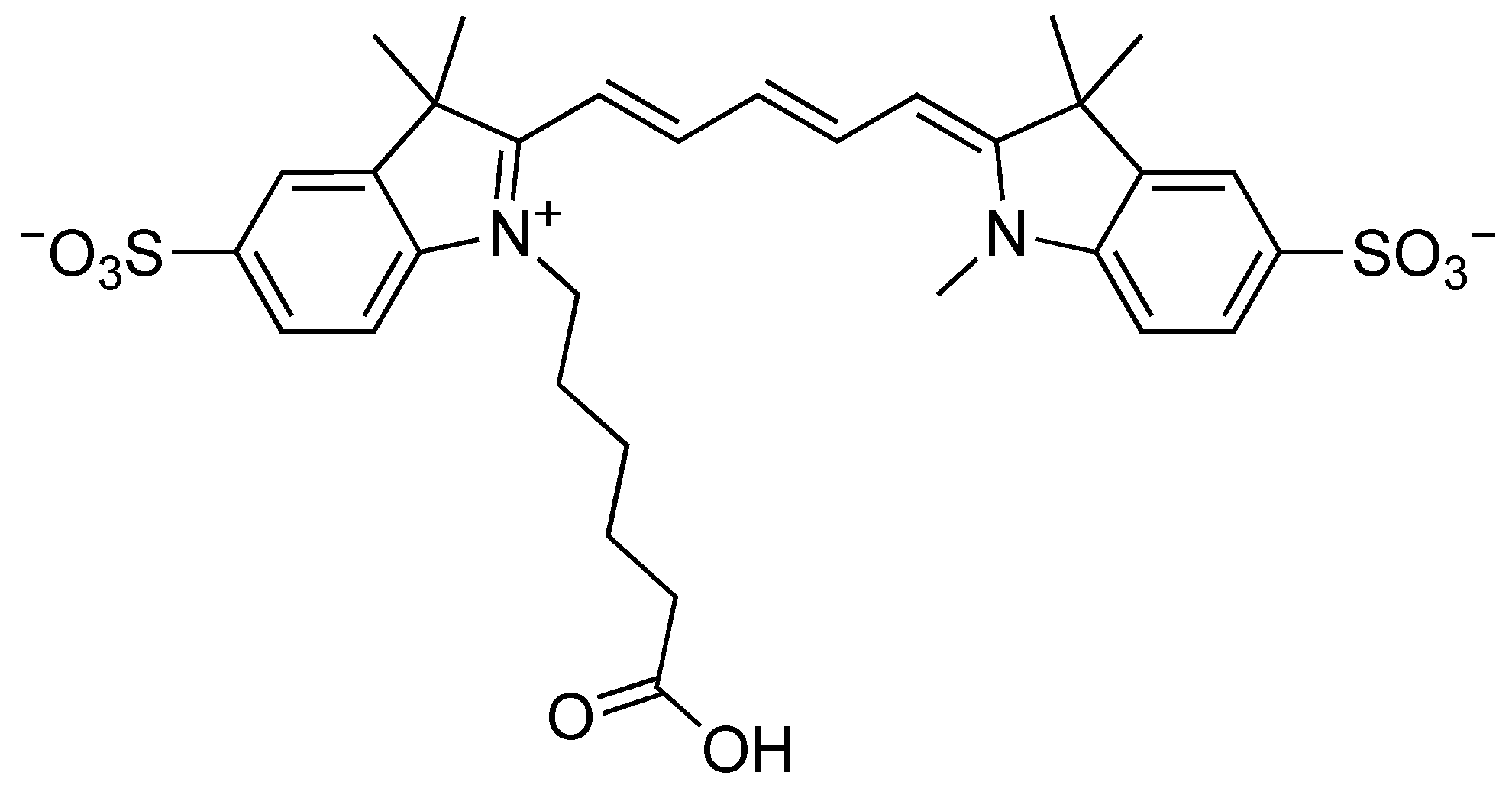


| Compound | UV–Vis Absorption | Fluorescence | |||
|---|---|---|---|---|---|
| λmax (nm) | log ε | λem (nm) | ΦF | ∆λ (nm) | |
| 1 | 651 | 4.99 | 672 | 0.30 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. A Sulfo-Cyanine Dye as a Colorimetric Chemosensor for Metal Cation Recognition. Eng. Proc. 2022, 27, 12. https://doi.org/10.3390/ecsa-9-13219
Martins CDF, Raposo MMM, Costa SPG. A Sulfo-Cyanine Dye as a Colorimetric Chemosensor for Metal Cation Recognition. Engineering Proceedings. 2022; 27(1):12. https://doi.org/10.3390/ecsa-9-13219
Chicago/Turabian StyleMartins, Cátia D. F., M. Manuela M. Raposo, and Susana P. G. Costa. 2022. "A Sulfo-Cyanine Dye as a Colorimetric Chemosensor for Metal Cation Recognition" Engineering Proceedings 27, no. 1: 12. https://doi.org/10.3390/ecsa-9-13219
APA StyleMartins, C. D. F., Raposo, M. M. M., & Costa, S. P. G. (2022). A Sulfo-Cyanine Dye as a Colorimetric Chemosensor for Metal Cation Recognition. Engineering Proceedings, 27(1), 12. https://doi.org/10.3390/ecsa-9-13219







