Human Periodontal Ligament Characterization by Means of Vibrational Spectroscopy and Electron Microscopy †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Techniques
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. FT-IR Microspectroscopy
2.2.3. Raman Microspectroscopy
3. Results and Discussion
3.1. SEM Observations
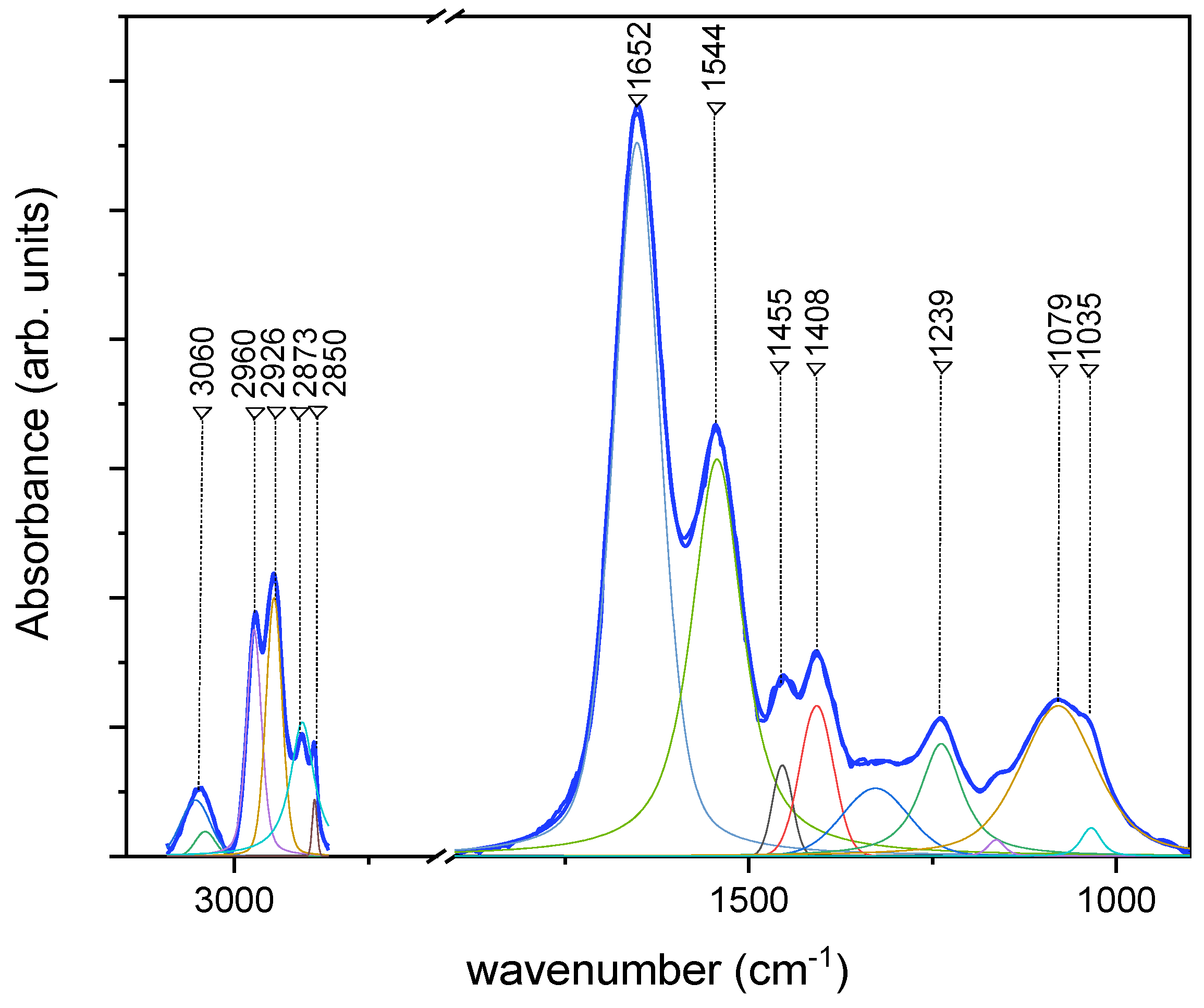
3.2. FT-IR Results
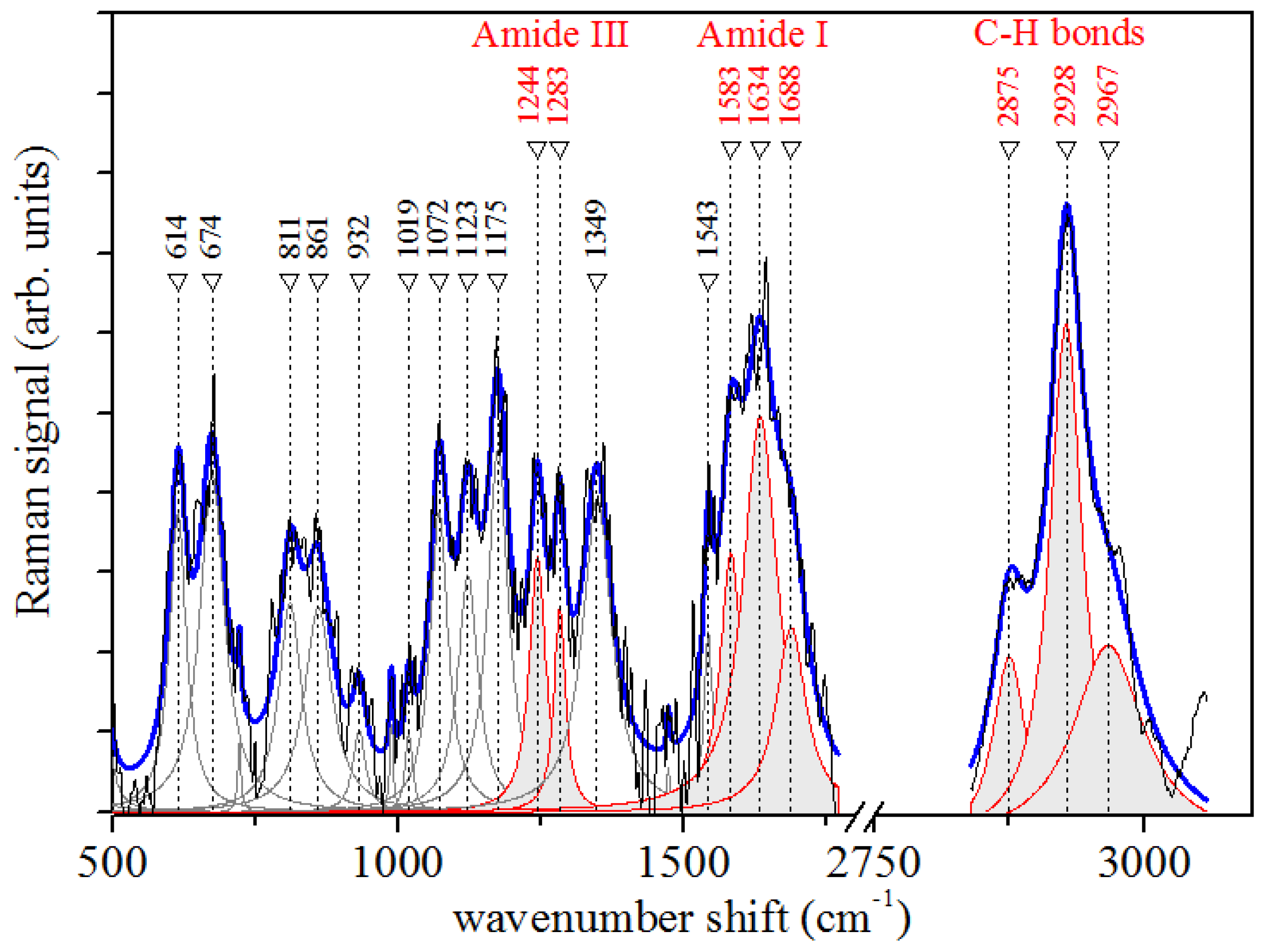
3.3. Raman Microspectroscopy Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anastasi, G.; Cordasco, G.; Matarese, G.; Rizzo, G.; Nucera, R.; Mazza, M.; Militi, A.; Portelli, M.; Cutroneo, G.; Favaloro, A. An immunohistochemical, histological, and electron-microscopic study of the human periodontal ligament during orthodontic treatment. J. Mol. Med. 2008, 21, 545–554. [Google Scholar] [CrossRef]
- d’Apuzzo, F.; Cappabianca, S.; Ciavarella, D.; Monsurrò, A.; Silvestrini-Biavati, A.; Perillo, L. Biomarkers of Periodontal tissue remodeling during orthodontic tooth movement in mice and men: Overview and clinical relevance. Sci. World J. 2013, 2013, 105873. [Google Scholar]
- Portaccio, M.; d’Apuzzo, F.; Perillo, L.; Grassia, V.; Errico, S.; Lepore, M. Infrared microspectroscopy characterization of gingival crevicular fluid during orthodontic treatment. J. Mol. Struct. 2019, 1176, 847–854. [Google Scholar] [CrossRef]
- Delfino, I.; Perna, G.; Lasalvia, M.; Capozzi, V.; Manti, L.; Camerlingo, C.; Lepore, M. Visible micro-Raman spectroscopy of single human mammary epithelial cells exposed to X-ray radiation. J. Biomed. Opt. 2015, 20, 035003. [Google Scholar] [CrossRef] [PubMed]
- Perillo, L.; D’Apuzzo, F.; Illario, M.; Laino, L.; Di Spigna, G.; Lepore, M.; Camerlingo, C. Monitoring Biochemical and structural changes in human periodontal ligaments during orthodontic treatment by means of micro-Raman spectroscopy. Sensors 2020, 20, 497. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; d’Apuzzo, F.; Grassia, V.; Perillo, L.; Lepore, M. Micro-Raman spectroscopy for monitoring changes in periodontal ligaments and gingival crevicular fluid. Sensor 2008, 14, 22552–22563. [Google Scholar] [CrossRef] [PubMed]

| FT-IR Mode ν (cm−1) | Assignments |
|---|---|
| 3279 | Amide A (-N-H ν) |
| 2960 | CH3 as. ν |
| 2926 | CH2 as. ν |
| 2873 | CH3 s. ν |
| 2850 | CH2 s. ν |
| 1652 | Amide I (C=O ν, C-N ν) |
| 1544 | Amide II (C-N ν, C-NH δ, α-helix) |
| 1455 | CH3 as. δ, CH2 s. |
| 1408 | COO− s. ν |
| 1239 | PO2− as. ν C-O-P ν |
| 1079 | PO2− s. ν C-O-P ν |
| 1035 | C-O ν |
| Raman Mode ν (cm−1) | Assignments |
|---|---|
| 614 | phosphate δ |
| 811 | CC skeleton |
| 861 | proline |
| 932 | phosphate |
| 1019 | C-N proline |
| 1072 | C-N proline |
| 1123 | C-N |
| 1175 | C-O ν |
| 1244 | random coil—Amide III |
| 1283 | α-helix—Amide III |
| 1349 | CH2 Glycine—Amide III |
| 1543 | N-H δ—Amide II |
| 1583 | β-sheet—Amide I |
| 1634 | α-helix—Amide I |
| 1688 | β-turn—Amide I |
| 2875 | CH2 as. ν |
| 2928 | CH3 s. ν |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camerlingo, C.; D’Apuzzo, F.; Cammarota, M.; Errico, S.; Portaccio, M.; Perillo, L.; Lepore, M. Human Periodontal Ligament Characterization by Means of Vibrational Spectroscopy and Electron Microscopy. Eng. Proc. 2020, 2, 35. https://doi.org/10.3390/ecsa-7-08176
Camerlingo C, D’Apuzzo F, Cammarota M, Errico S, Portaccio M, Perillo L, Lepore M. Human Periodontal Ligament Characterization by Means of Vibrational Spectroscopy and Electron Microscopy. Engineering Proceedings. 2020; 2(1):35. https://doi.org/10.3390/ecsa-7-08176
Chicago/Turabian StyleCamerlingo, Carlo, Fabrizia D’Apuzzo, Marcella Cammarota, Sonia Errico, Marianna Portaccio, Letizia Perillo, and Maria Lepore. 2020. "Human Periodontal Ligament Characterization by Means of Vibrational Spectroscopy and Electron Microscopy" Engineering Proceedings 2, no. 1: 35. https://doi.org/10.3390/ecsa-7-08176
APA StyleCamerlingo, C., D’Apuzzo, F., Cammarota, M., Errico, S., Portaccio, M., Perillo, L., & Lepore, M. (2020). Human Periodontal Ligament Characterization by Means of Vibrational Spectroscopy and Electron Microscopy. Engineering Proceedings, 2(1), 35. https://doi.org/10.3390/ecsa-7-08176






