Development of a Compact Optical Measurement System to Quantify the Optical Properties of Fluorescently Labeled Cervical Cancer Cells †
Abstract
:1. Introduction
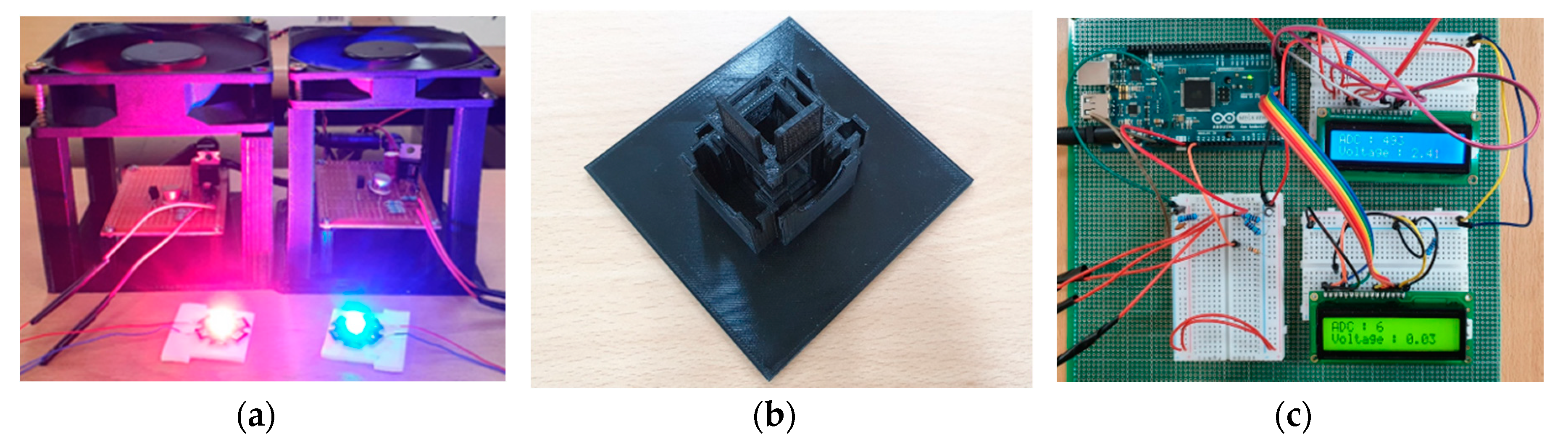
2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dean, P.N. Overview of Flow Cytometry Instrumentation. Curr. Protoc. Cytom. 2007, 39, 1.1.1–1.1.8. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.J. Principles and Applications of Flow Cytometry and Cell Sorting in Companion Animal Medicine. Vet. Clin. Small Anim. Pract. 2012, 42, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hoffman, R.A. Standardization, Calibration, and Control in Flow Cytometry. Curr. Protoc. Cytom. 2017, 79, 1.3.1–1.3.27. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Cho, K.; Seo, J.; Heo, G.; Choe, S. An Alternative Approach to Detecting Cancer Cells by Multi-Directional Fluorescence Detection System Using Cost-Effective LED and Photodiode. Sensors 2019, 19, 2301. [Google Scholar] [CrossRef] [PubMed]
| Fluorescent Dye | Slope [mV/#Cell] | |
|---|---|---|
| Calcein-AM | 22.64 | 0.933 |
| DiD | 7.61 | 0.954 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Oh, J.-H.; Choe, S.-w. Development of a Compact Optical Measurement System to Quantify the Optical Properties of Fluorescently Labeled Cervical Cancer Cells. Eng. Proc. 2020, 2, 15. https://doi.org/10.3390/ecsa-7-08256
Lee H, Oh J-H, Choe S-w. Development of a Compact Optical Measurement System to Quantify the Optical Properties of Fluorescently Labeled Cervical Cancer Cells. Engineering Proceedings. 2020; 2(1):15. https://doi.org/10.3390/ecsa-7-08256
Chicago/Turabian StyleLee, Hun, Ji-Hyeon Oh, and Se-woon Choe. 2020. "Development of a Compact Optical Measurement System to Quantify the Optical Properties of Fluorescently Labeled Cervical Cancer Cells" Engineering Proceedings 2, no. 1: 15. https://doi.org/10.3390/ecsa-7-08256
APA StyleLee, H., Oh, J.-H., & Choe, S.-w. (2020). Development of a Compact Optical Measurement System to Quantify the Optical Properties of Fluorescently Labeled Cervical Cancer Cells. Engineering Proceedings, 2(1), 15. https://doi.org/10.3390/ecsa-7-08256






