Abstract
The consumption of water contaminated with bacteria can lead to foodborne disease outbreaks. For this reason, the development of rapid and sensitive analytical methods for bacteria detection is of primary importance for public health protection. Here, a miniaturized immunosensor based on Mach–Zehnder Interferometry for the simultaneous, real-time determination of S. typhimurium and E. coli in drinking water is presented. For the assay, mixtures of bacteria solutions with anti-bacteria-specific antibodies were run over the chip, followed by biotinylated anti-species-specific antibody and streptavidin solutions. The assay was fast (10 min), accurate, sensitive (LOD: 3 × 102 cfu/mL for S. typhimurium; 2 × 102 cfu/mL for E. coli) and reproducible. The analytical characteristics achieved combined with the small chip size make the proposed biosensor suitable for on-site bacteria determination in drinking water samples.
1. Introduction
The consumption of food and water contaminated with pathogens is of global interest as it leads to 48 × 106 infections annually, resulting in 128,000 hospitalizations and 3000 deaths [1]. According to CDC, the estimated incidents of foodborne illness caused by 31 pathogenic bacteria in the US amount to a total of 9 million cases per year, from which 20% is attributed to Salmonella spp., Escherichia coli O157:H7, Staphylococcus aureus, Clostridium perfringens, Campylobacter spp. and Shigella spp. [2]. Among them, Salmonella typhimurium (S. typhimurium) and Escherichia coli O157:H7 (E. coli O157:H7) are both facultatively anaerobic, rod-shaped, Gram-negative bacteria, belonging to the Enterobacteriaceae family and are most frequently associated with foodborne illnesses. The ingestion of S. typhimurium causes fever, nausea, diarrhea, stomach discomfort, vomiting, dehydration and weakness, while E. coli may cause, on top of the aforementioned symptoms, potentially life-threatening complications known as hemolytic uremic syndrome and hemorrhagic colitis. In both cases, the clinical symptoms may last from 5 to 7 days [3,4]. The number of outbreaks due to S. typhimurium and E. coli O157:H7 infections in combination with the economic loss associated with these foodborne illnesses due to medical costs, loss of work hours and product recalls have imposed the need for rapid diagnostic methods for pathogen detection [5].
The conventional methods for bacteria detection and identification are based on culturing and plating. Those methods are reliable but include several steps, such as pre-enrichment, selective enrichment, isolation and confirmation through biochemical and serological tests, which are rather time consuming and require at least 5–7 days to complete. In order to shorten the analysis time to 2–4 days, ELISA- and DNA-based methods have been employed for bacteria identification, thus replacing the selective plating steps [6,7].
In recent years, biosensors based on electrochemical, piezoelectric or optical transducers are gaining ground in foodborne bacteria detection. Concerning electrochemical immunosensors, devices employing amperometric, potentiometric, impedemetric and conductimetric detection principles have been developed for detection of bacteria [8,9,10]. Although these sensors claim inexpensive analysis and potential for miniaturization, they often require labels for signal enhancement to improve detection limits. Similarly, immunosensors based on piezoelectric phenomena are capable of label-free detection but they lack in sensitivity [11]. On the other hand, optical biosensors utilizing different transduction principles, such as light absorbance, SPR, fluorescence, light polarization and Raman scattering, are powerful tools for foodborne bacteria detection [12]. Optical detection provides several advantages over other transduction principles, such as less interference from the sample and ability for direct determination of pathogens in complex matrices with minimal sample treatment. Although SPR biosensors are widely used for label-free bacteria detection, their limit of detection is usually higher than 103 cfu/mL, [13,14]. Among the label-free biosensors, interferometric ones are the most promising bacteria detection systems, as they offer high sensitivity and multiplexing capability for real-time determinations. Recently, a bi-modal interferometric sensor, an interferometric reflectance imaging sensor and a microcavity in-line Mach–Zehnder interferometer have been employed for the detection of E. coli with detection limits of 40, 2.2 and 100 cfu/mL, respectively [15,16,17]. Moreover, another interferometric sensor based on white light reflectance spectroscopy has been developed for the detection of S. typhimurium in drinking water samples, exhibiting a detection limit of 320 cfu/mL [18].
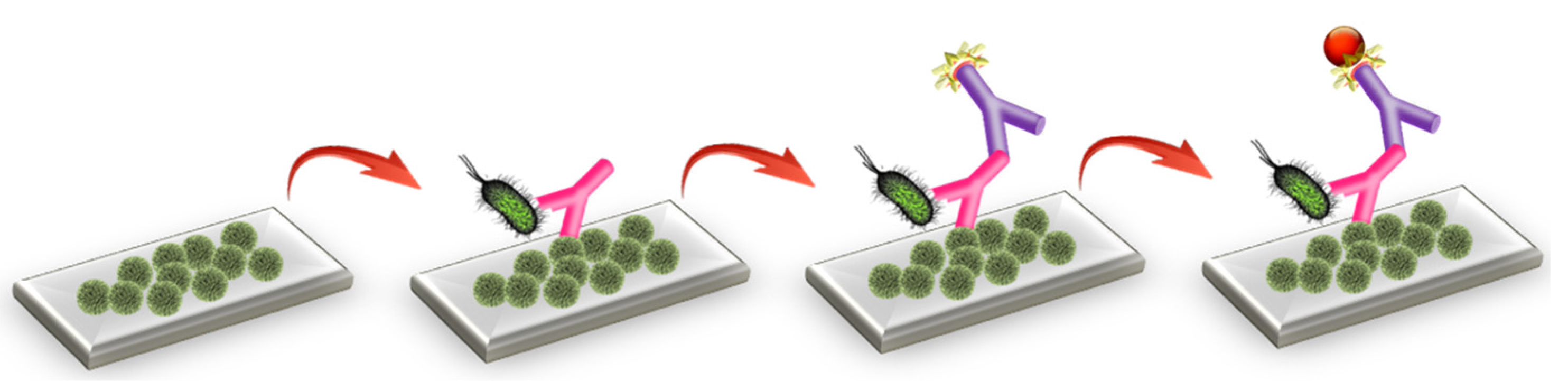
In this work, a label-free optical immunosensor based on arrays of Mach–Zehnder interferometers (MZIs) monolithically integrated onto silicon chips, which are appropriate for multi-analyte determinations, is employed. The MZIs chips have been successfully utilized for the determination of allergens and mycotoxins in foodstuffs, as well as for the detection of goat milk and PDO cheeses adulteration with bovine milk [19,20,21,22]. Here, the MZIs chips are employed, for the first time, for the simultaneous determination of S. typhimurium and E. coli in drinking water samples. The detection of bacteria was based on the competitive immunoassay principle through biofunctionalization of the sensing arm of the MZIs with the lipopolysaccharides (LPS) of the two bacteria (Figure 1). Biomolecular reactions on the LPS modified sensing arm change the effective refractive index, causing a blue shift of the interference spectrum. The spectral shift is transformed to phase shift, and the signal is expressed in radians. Several assay parameters were optimized aiming for fast and sensitive simultaneous determination of both bacteria in drinking water.
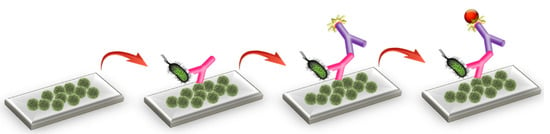
Figure 1.
Three-dimensional schematic of assay configuration for bacteria detection using the MZI sensor.
2. Experimental Section
2.1. Materials
Salmonella enterica serovar typhimurium (S. typhimurium, ATCC 14028) and Escherichia coli O157:H7 (E. coli O157:H7, NCTC 12900) were kindly provided from Delta Foods S.A. (Athens, Greece). E. coli LPS was obtained from Creative Diagnostics (Upton, NY, USA). The goat polyclonal antibody against E. coli LPS was from Kirkegaard & Perry Lab Inc. (Gaithersburg, MD, USA). The rabbit polyclonal antibody against S. typhimiurim LPS, donkey anti-goat IgG antibody and donkey anti-rabbit IgG antibody were purchased from Bio-Rad (Watford, UK). Salmonella LPS, bovine serum albumin (BSA) and 3-aminopropyl-triethoxysilane (APTES) were purchased from Sigma-Aldrich (Darmstadt, Germany). Streptavidin was from Thermo-Scientific (Waltham, MA, USA). The water used in the study was double distilled. Donkey anti-goat IgG and donkey anti-rabbit IgG antibodies were biotinylated according to a previously published protocol [22].
2.2. Chip Fabrication and Signal Processing
Fabrication of the chips was performed following mainstream silicon technology as described previously [21,22]. The chip consists of an array of ten silicon nitride MZIs, each one of them coupled with a respective silicon LED. The ten LEDs are serially turned on and off using a multiplexer. The chip is covered by a silicon oxide cladding layer that was selectively removed from a 600 μm long area over the sensing arm of each MZI to allow for interaction of the waveguided photons with the spotted biomolecules onto the sensing arm. The ten MZIs converge in a single output at the edge of the chip where the transmitted light is collected by an external spectrometer (QE65000, Ocean Optics, Orlando, FL, USA). The spectral shifts caused by the immunoreactions over the sensing arm of the MZIs are continuously recorded and converted to phase shifts through discrete Fourier transform.
2.3. Chemical and Biological Functionalization of the Chip
For the chemical activation, the chips were cleaned and hydrophilized through O2 plasma treatment for 30 s. Then, they were immersed for 2 min in a 0.5% (v/v) APTES solution, rinsed, dried under nitrogen stream and heated at 120 °C for 20 min. The biological activation of the chips was performed using the BioOdyssey Calligrapher Mini Arrayer. Hence, 3 MZIs per chip were spotted with 100 μg/mL of S. typhimurium LPS solution, 4 MZIs with 50 μg/mL of E. coli LPS solution and the remaining 3 with 100 μg/mL of BSA solution for the determination of non-specific binding. After the completion of spotting, the chips were incubated overnight at 4 °C in humidity chamber. Then, the biofunctionalized chips were washed and incubated for 1 h in 1% (w/v) BSA in 0.1 M NaHCO3 solution to block the non-specific binding sites on the sensing arm, rinsed with water and dried under nitrogen stream.
2.4. Immunoassay for Bacteria Detection with MZI Immunosensor
The delivery of the samples over the chip surface was achieved through attachment of an appropriate microfluidic module onto the chip. Then, the chip was placed on a handling frame and inserted in the docking station of the measuring device. Prior to assay, calibrators/samples were mixed with the antibodies against S. typhimurium and E. coli LPS at 1:1 volume ratio and incubated for 30 min. After chip equilibration with assay buffer, 100 μL of these mixtures was run over the chip at a rate of 35 μL/min, followed by 100 μL of biotinylated anti-species-specific antibodies and 100 μL of streptavidin solution (Figure 1). After assay completion, a regeneration step was followed in order to remove the bound antibodies from the biofunctionalized surface and reuse the chip for the next sample. Thus, 100 μL of 0.05 M HCl solution and 100 μL of 0.05 M NaOH solution were pumped over the chip sequentially, and finally, 100 μL of assay buffer was flown for chip equilibration.
3. Results
3.1. Optimization of Assay Parameters
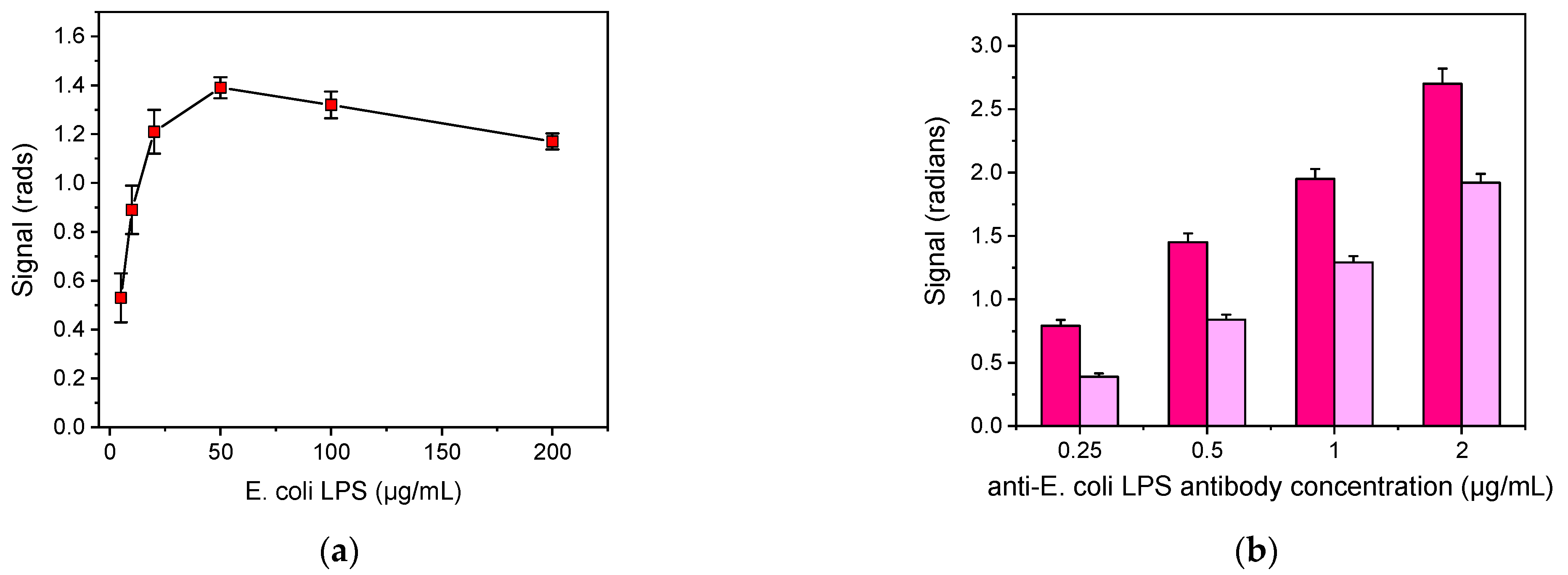
The detection of bacteria in drinking water samples was based on the competitive immunoassay principle. Thus, several parameters were optimized with respect to the maximum signal and the percent signal drop obtained for certain bacteria calibrators. Firstly, the optimum concentration of bacteria LPS for immobilization onto the sensor’s surface was determined by running for 1 h zero calibrators over chips spotted with LPS solutions with concentrations ranging from 5 to 200 μg/mL. As shown in Figure 2a, indicatively for E. coli LPS, the signal increased and reached a maximum at a concentration of 50 μg/mL, whereas for concentrations higher than 100 μg/mL the signal started to decline. Furthermore, the sensor-to-sensor and the chip-to-chip signal variation was significantly improved (CV < 5%) when the concentration of LPS used for coating was equal or higher than 50 μg/mL compared to the signal variations obtained from chips spotted with lower concentrations. Thus, 50 μg/mL concentration of E. coli LPS was selected for further experimentation. In a similar way, the optimum concentration for immobilization regarding the S. typhimurium LPS was determined to be 100 μg/mL.
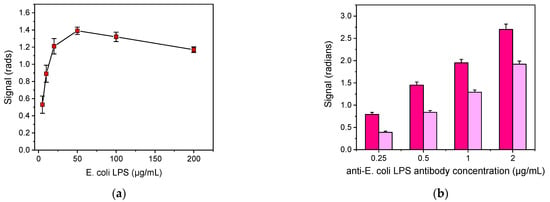
Figure 2.
(a) Signal values for zero calibrator vs. E. coli LPS concentrations used for coating and (b) signals corresponding to zero calibrator (magenta columns) or an E. coli LPS calibrator with concentration of 0.025 μg/mL (purple columns) obtained using different concentrations of anti-E. coli antibody. Each point is the mean value of ten waveguides per chip ± SD.
Another parameter optimized was the concentration of the anti-bacteria antibodies in order to select the one providing adequate signal in combination with detection sensitivity. The concentrations tested ranged from 0.25 to 2 μg/mL for E. coli LPS, and 0.5 to 3 μg/mL for S. typhimurium. As presented in Figure 2b, indicatively for E. coli, adequate signal (≥1 rad) was achieved for anti-E. coli LPS concentrations ≥0.5 μg/mL. However, although higher signals were obtained by increasing the antibody concentration, the higher detection sensitivity was achieved for antibody concentration of 0.5 μg/mL. For S. typhimurium, the optimum antibody concentration was 1 μg/mL.
3.2. Matrix Effect
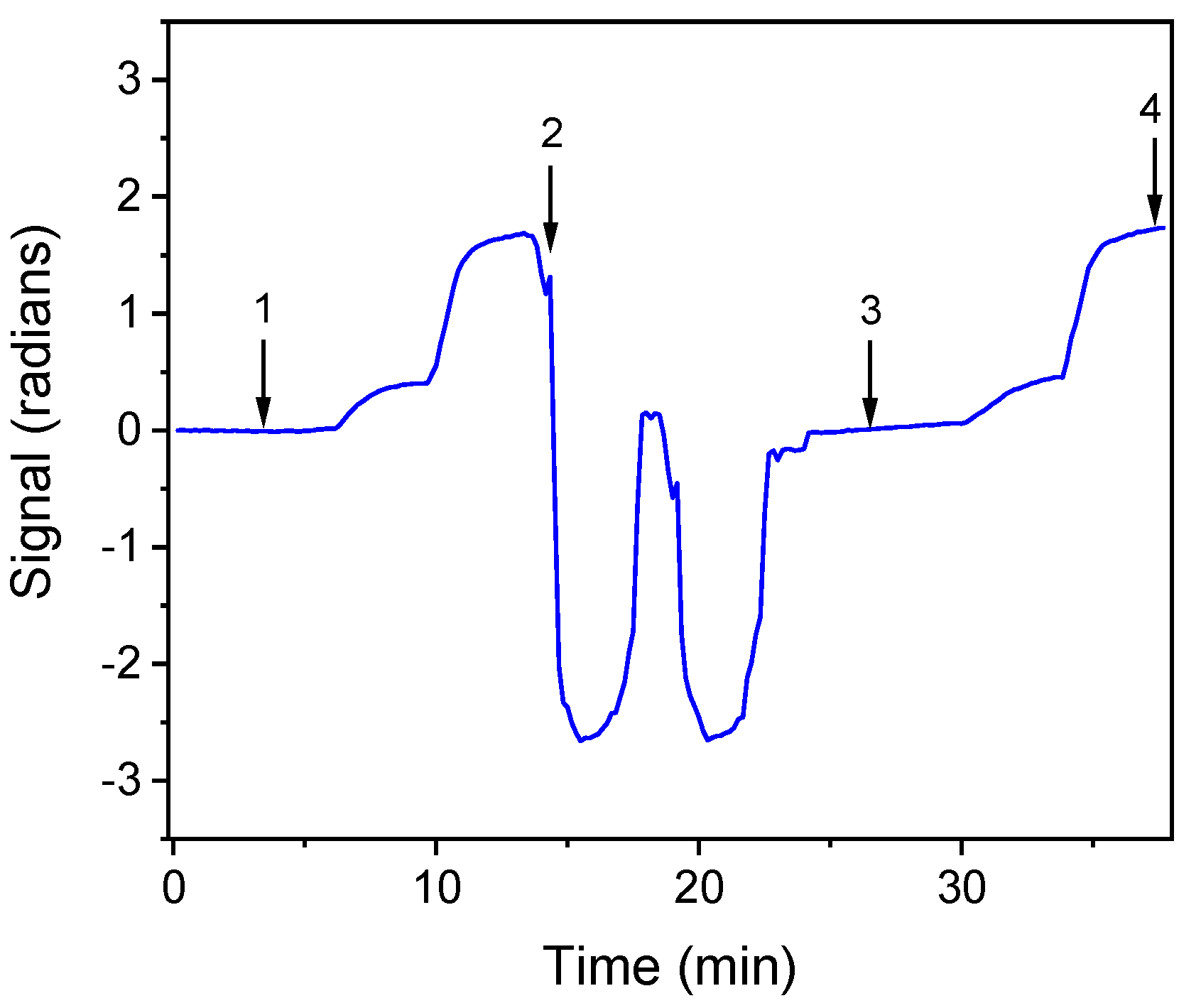
For the determination of bacteria in drinking water samples, the effect of tap water on the signal was investigated. For this reason, E. coli and S. typhimurium zero calibrators were prepared in assay buffer as well as in tap water. As shown in Figure 3, indicatively for S. typhimurium, the signal obtained from zero calibrator prepared in tap water was similar to that of the zero calibrator prepared in assay buffer. Moreover, the calibration curves obtained with calibrators prepared in both matrices were almost identical. Thus, the calibrators were prepared in assay buffer.
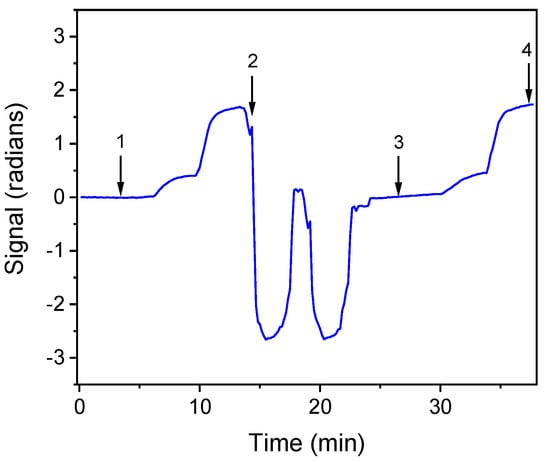
Figure 3.
Real-time response obtained for S. typhimurium zero calibrator prepared in: assay buffer (arrow 1 to 2); regeneration and equilibration (arrow 2 to 3); and zero calibrator in tap water (arrow 3 to 4).
3.3. Analytical Characteristics and Calibration Curves Using the MZI Chip
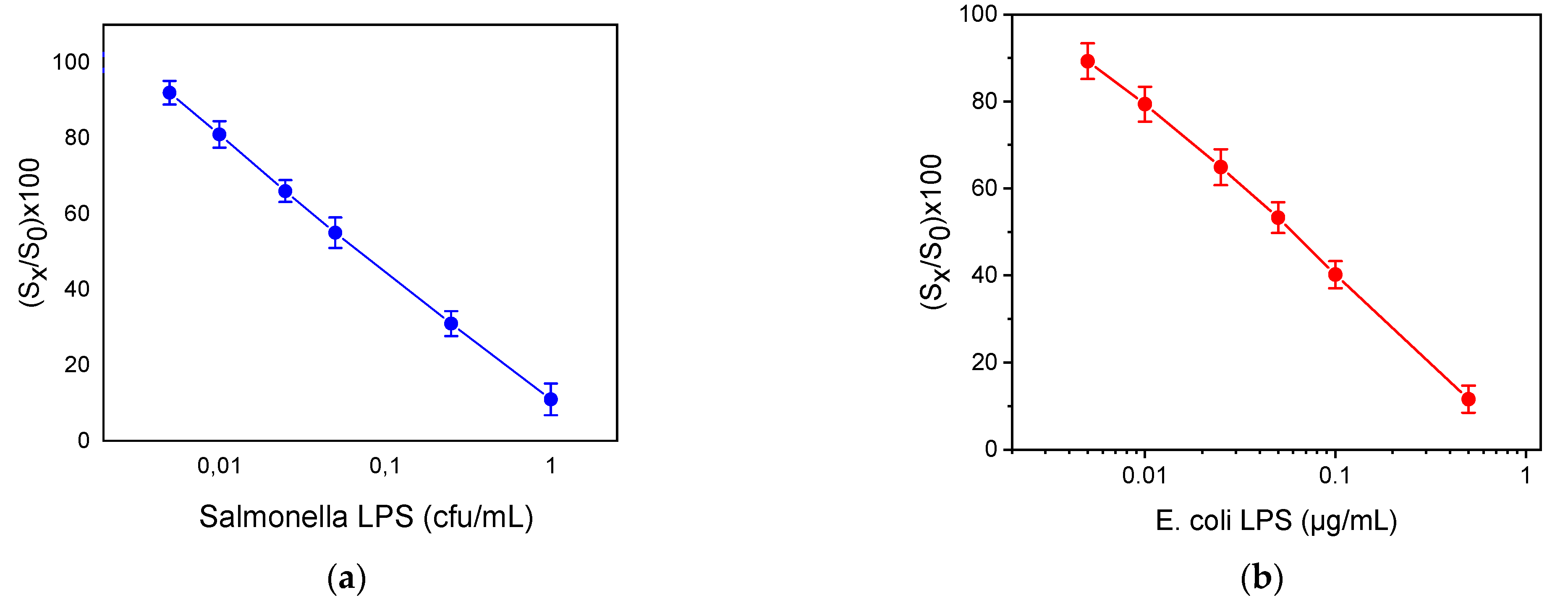
In Figure 4, the calibration curves for S. typhimurium and E. coli LPS are provided. The dynamic range of S. typhimurium and E. coli LPS assays ranged from 0.005 to 1 μg/mL and from 0.005 to 0.5 μg/mL, respectively. The limit of detection of the assays was determined as the concentration corresponding to signal equal to -3SD of the mean zero calibrator signals (28 replicate values from 4 chips; 7 MZIs per chip) and was 0.004 μg/mL for both bacteria. Moreover, the limit of detection was 3 × 102 cfu/mL and 2 × 102 cfu/mL for S. typhimurium and E. coli, respectively. The accuracy of the assay was also determined through recovery experiments. For this reason, tap water was spiked with three different bacteria concentrations. The recovery values ranged from 91 to 112%, indicating the high accuracy of the assay performed using the MZI chip. The repeatability of the assay was determined using tap water samples spiked with four different concentrations of the bacteria. The intra-assay coefficients of variation (CVs) were calculated after repetitive measurements of the tap water samples during the same day, whereas the inter-assay CVs were determined by measuring the tap water samples in seven different days in a period of one month and were less than 5% and 7%, respectively.
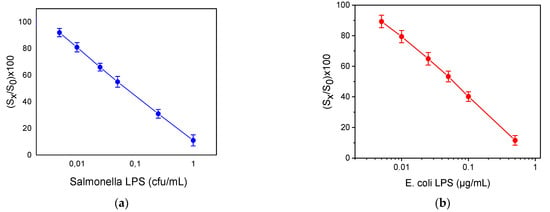
Figure 4.
Calibration curves of (a) S. typhimurium LPS and (b) E. coli LPS. (Sx/S0) × 100 represents the percent ratio of each calibrator signal (Sx) to the zero calibrator signal (S0). Each point is the mean value of seven waveguides per chip ± SD.
4. Conclusions
The simultaneous determination of S. typhimurium and E. coli in drinking water using the MZI immunosensor chips was presented. The sensor provided real-time detection of the two bacteria in 10 min, employing a three-step assay configuration. The assay was accurate, repeatable and sensitive with detection limits at the order of 102 cfu/mL. Thus, it is expected that the proposed sensor could find wide application in Drinking Water Distribution System and in low-resources environment for the fast on-site monitoring of bacteria.
Author Contributions
Conceptualization, M.A., P.P., I.R., K.M. and S.K.; M.A. and P.P.; formal analysis, M.A.; investigation, M.A.; resources, K.M. and I.R.; data curation, M.A. and S.K.; writing—original draft preparation, M.A.; writing—review and editing, P.P., K.M. and I.R.; visualization, M.A.; supervision, P.P. and S.K.; project administration, S.K.; funding acquisition, M.A. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
M.A. was supported by the program of Industrial Scholarships of Stavros Niarchos Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/foodborneburden/estimates-overview.html (accessed on 30 March 2022).
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chen, B. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food Drug Anal. 2016, 24, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waswa, J.W.; Debroy, C.; Irudayaraj, J. Rapid detection of Salmonella enteritidis and Escherichia coli using Surface Plasmon Resonance biosensor. J. Food Process Eng. 2006, 29, 373–385. [Google Scholar] [CrossRef]
- Xu, L.; Bai, X.; Bhunia, A.K. Current state of development of biosensors and their application in foodborne pathogen detection. J. Food Prot. 2021, 84, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.I.; McQuillan, J.; Taiwo, M.; Parks, R.; Stenton, C.A.; Morgan, H.; Mowlem, M.C.; Lees, D.N. A highly specific Escherichia coli qPCR and its comparison with existing methods for environmental waters. Water Res. 2017, 126, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Liu, L.; Song, S.; Tang, L.; Kuang, H.; Xu, C. A highly sensitive ELISA and immunochromatographic strip for the detection of Salmonella typhimurium in milk samples. Sensors 2015, 15, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Zelada-Guillen, G.A.; Bhosale, S.V.; Riu, J.; Rius, F.X. Real-time potentiometric detection of bacteria in complex samples. Anal. Chem. 2010, 82, 9254–9260. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Li, Y. An electrochemical biosensor for rapid detection of E. coli O157:H7 with highly efficient bifunctional glucose oxidase-polydopamine nanocomposites and Prussian blue modified screen-printed interdigitated electrodes. Analyst 2016, 141, 5441–5449. [Google Scholar] [CrossRef] [Green Version]
- Subjakova, V.; Oravczova, V.; Tatarko, M.; Hianik, T. Advances in electrochemical aptasensors and immunosensors for detection of bacterial pathogens in food. Electrochim. Acta 2021, 389, 138724. [Google Scholar] [CrossRef]
- Salam, F.; Uludag, Y.; Tothill, I.E. Real-time and sensitive detection of Salmonella typhimurium using an automated quartz crystal microbalance (QCM) instrument with nanoparticles amplification. Talanta 2013, 115, 761–767. [Google Scholar] [CrossRef]
- Terry, L.A.; White, S.F.; Tigwell, L.J. The application of biosensors to fresh produce and the wider food industry. J. Agric. Food Chem. 2005, 53, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, Z.; Chen, J.; Huang, Z.; Wang, X.; An, H.; Duan, Y. Novel W-shaped fiber-optic probe-based localized surface Plasmon resonance biosensor for real-time detection of Salmonella typhimurium. Anal. Chem. 2018, 90, 13640–13646. [Google Scholar] [CrossRef] [PubMed]
- Kaushika, S.; Tiwaria, U.K.; Pala, S.S.; Sinhaa, R.K. Rapid detection of Escherichia coli using fiber optic surface plasmon resonance immunosensor based on biofunctionalized Molybdenum disulfide (MoS2) nanosheets. Biosens. Bioelectron. 2019, 126, 501–509. [Google Scholar] [CrossRef]
- Maldonado, J.; González-Guerrero, A.B.; Domínguez, C.; Lechuga, L.M. Label-free bimodal waveguide immunosensor for rapid diagnosis of bacterial infections in cirrhotic patients. Biosens. Bioelectron. 2016, 85, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zaraee, N.; Kanik, F.E.; Bhuiya, A.M.; Gong, E.S.; Geib, M.T.; Ünlü, N.L.; Ozkumur, A.Y.; Dupuis, J.R.; Ünlü, M.S. Highly sensitive and label-free digital detection of whole cell E. coli with Interferometric Reflectance Imaging. Biosens. Bioelectron. 2020, 162, 112258. [Google Scholar] [CrossRef] [PubMed]
- Janik, M.; Koba, M.; Celebanska, A.; Bock, W.J.; Smietana, M.; Live, E. coli bacteria label-free sensing using a microcavity in-line Mach-Zehnder interferometer. Sci. Rep. 2018, 8, 17176. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Tzialla, K.; Voulgari, A.; Dikeoulia, M.; Raptis, I.; Kakabakos, S.E.; Petrou, P. Rapid Detection of Salmonella typhimurium in drinking water by a White Light Reflectance Spectroscopy immunosensor. Sensors 2021, 21, 2683. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Petrou, P.S.; Makarona, E.; Haasnoot, W.; Moser, I.; Jobst, G.; Goustouridis, D.; Lees, M.; Kalatzi, K.; Raptis, I.; et al. Ultrafast multiplexed-allergen detection through advanced fluidic design and monolithic interferometric silicon chips. Anal. Chem. 2018, 90, 9559–9567. [Google Scholar] [CrossRef]
- Pagkali, V.; Petrou, P.S.; Makarona, E.; Peters, J.; Haasnoot, W.; Jobst, G.; Moser, I.; Gajos, K.; Budkowski, A.; Economou, A.; et al. Simultaneous determination of aflatoxin B 1, fumonisin B 1 and deoxynivalenol in beer samples with a label-free monolithically integrated optoelectronic biosensor. J. Hazard. Mater. 2018, 359, 445–453. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Botsialas, A.; Salapatas, A.; Petrou, P.S.; Haasnoot, W.; Makarona, E.; Gerhard, J.; Goustouridis, D.; Siafaka-Kapadai, A.; Raptis, I.; et al. Assessment of goat milk adulteration with a label-free monolithically integrated optoelectronic biosensor. Anal. Bioanal. Chem. 2015, 407, 3995–4004. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Petrou, P.S.; Raptis, I.; Misiakos, K.; Livaniou, E.; Makarona, E.; Kakabakos, S. Rapid detection of mozzarella and feta cheese adulteration with cow milk through a silicon photonic immunosensor. Analyst 2021, 146, 529. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).