Flexible Water-Activated Battery on a Polyester–Cotton Textile †
Abstract
:1. Introduction
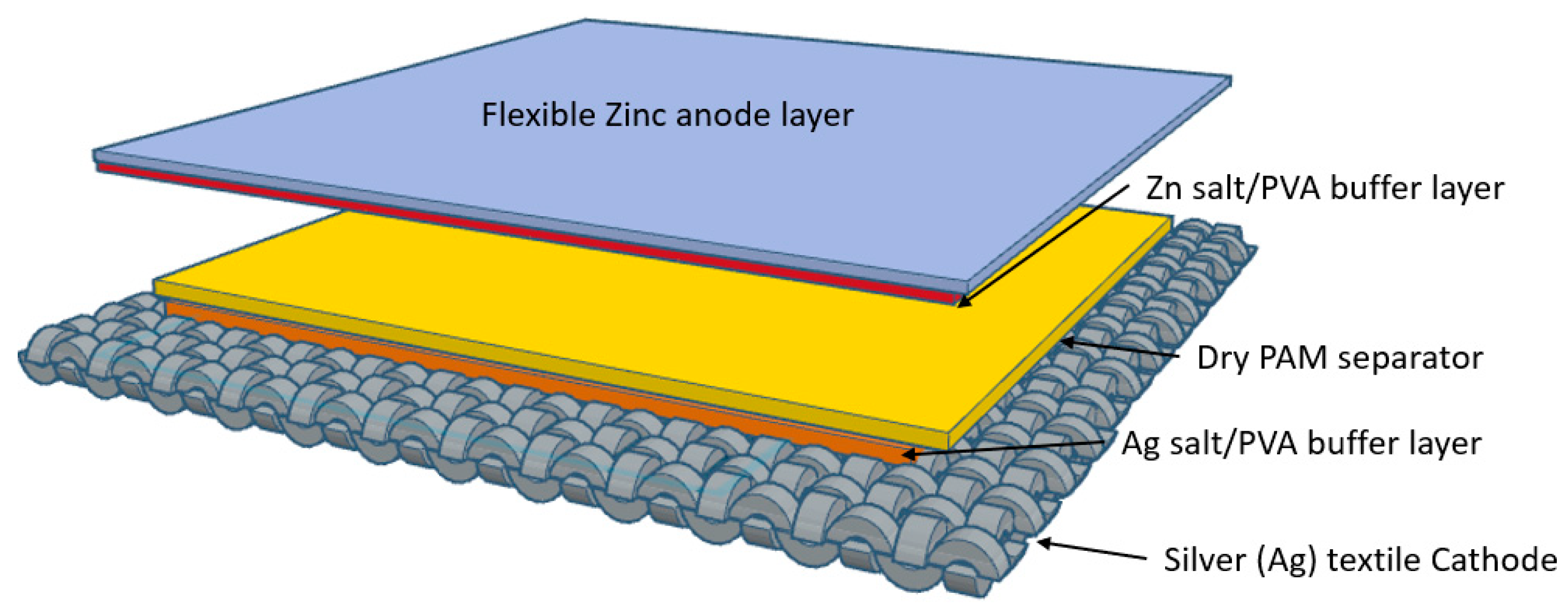
2. Material and Methods
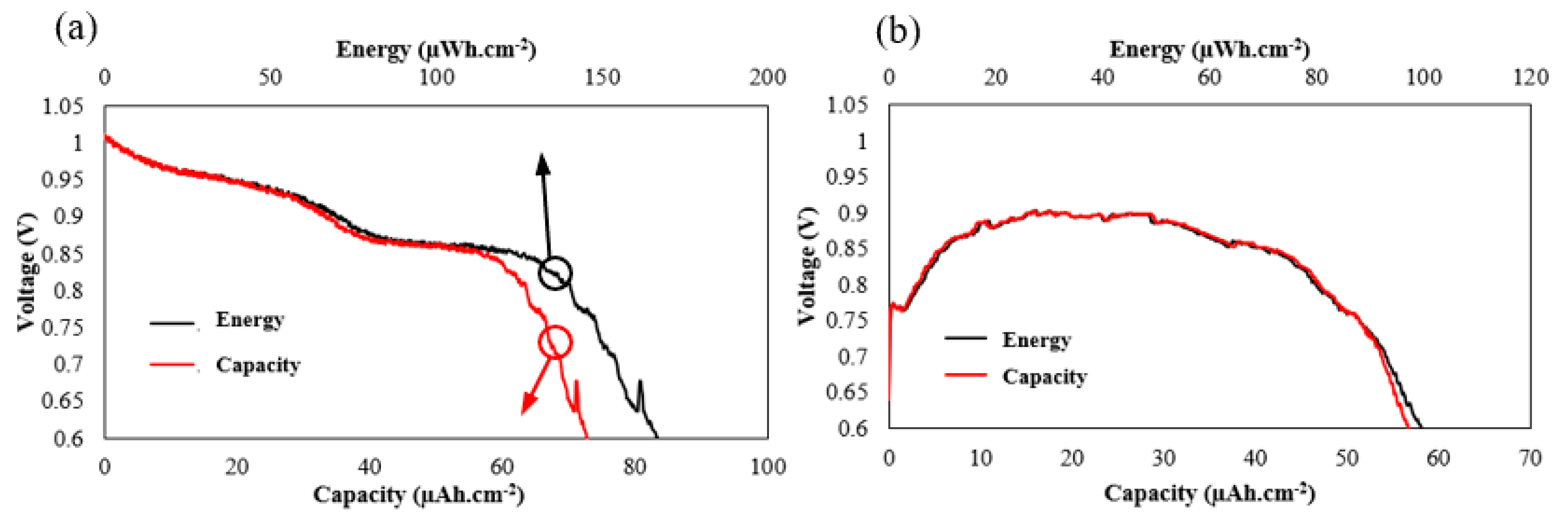
3. Results
4. Discussion and Concussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vališevskis, A.; Briedis, U.; Carvalho, M. Development of flexible textile aluminium-air battery prototype. Mater. Renew. Sustain. Energy 2021, 10, 1–6. [Google Scholar] [CrossRef]
- Mauriello, M.; Gubbels, M.; Froehlich, J.E. Social fabric fitness: The design and evaluation of wearable E-textile displays to support grouprunning. In Proceedings of the SIGCHI Conferences on Human Factors in Computing Systems, Toronto, ON, Canada, 26 April–1 May 2014; pp. 2833–2842. [Google Scholar] [CrossRef]
- Nguyen, D.; Ito, Y.; Taguchi, K. A water-activated paper-based battery based on activated carbon powder anode and CuCl2/CNT cathode. Energy Rep. 2020, 6, 215–219. [Google Scholar] [CrossRef]
- Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2001; Chapter 16. [Google Scholar]
- Liu, X.; Lillehoj, P.B. A liquid-activated textile battery for wearable biosensors. J. Physics Conf. Ser. 2015, 660, 012063. [Google Scholar] [CrossRef]
- Vilkhu, R.; Thio, W.; Das Ghatak, P.; Sen, C.K.; Co, A.; Kiourti, C. Power generation for wearable electronics: Designing electrochemical storage on fabrics. IEEE Access 2018, 6, 28945–28950. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yong, S.; Hillier, N.; Arumugam, S.; Beeby, S. Screen Printed Flexible Water Activated Battery on Woven Cotton Textile as a Power Supply for E-Textile Applications. IEEE Access 2020, 8, 206958–206965. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, S.; Hillier, N.; Beeby, S. Flexible Water-Activated Battery on a Polyester–Cotton Textile. Eng. Proc. 2022, 15, 20. https://doi.org/10.3390/engproc2022015020
Yong S, Hillier N, Beeby S. Flexible Water-Activated Battery on a Polyester–Cotton Textile. Engineering Proceedings. 2022; 15(1):20. https://doi.org/10.3390/engproc2022015020
Chicago/Turabian StyleYong, Sheng, Nick Hillier, and Stephen Beeby. 2022. "Flexible Water-Activated Battery on a Polyester–Cotton Textile" Engineering Proceedings 15, no. 1: 20. https://doi.org/10.3390/engproc2022015020
APA StyleYong, S., Hillier, N., & Beeby, S. (2022). Flexible Water-Activated Battery on a Polyester–Cotton Textile. Engineering Proceedings, 15(1), 20. https://doi.org/10.3390/engproc2022015020






