ZnO and TiO2 Assisted Photocatalytic Degradation of Butachlor in Aqueous Solution under Visible Light †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
3. Results and Discussion
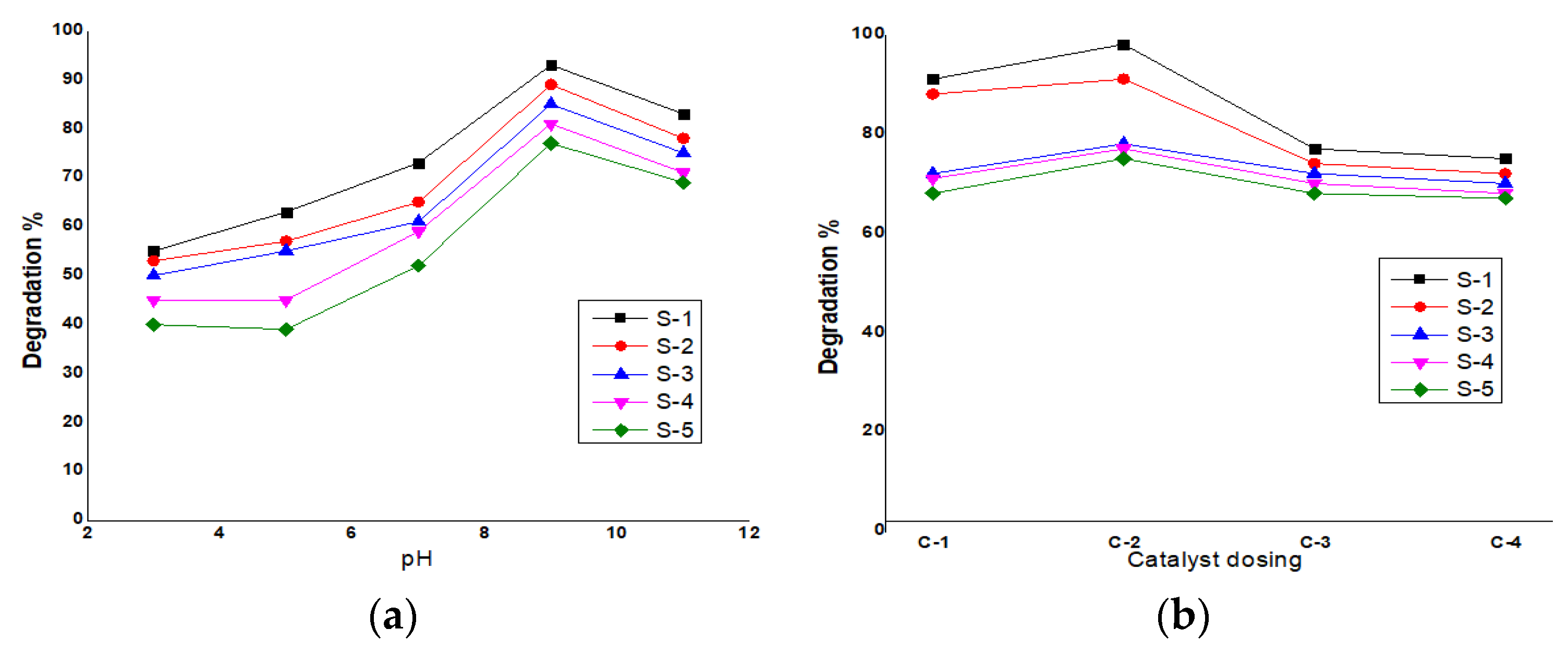
3.1. TiO2 Degradation Efficiency at Different pH
3.2. TiO2 Catalyst Degradation Efficiency at Different Doses
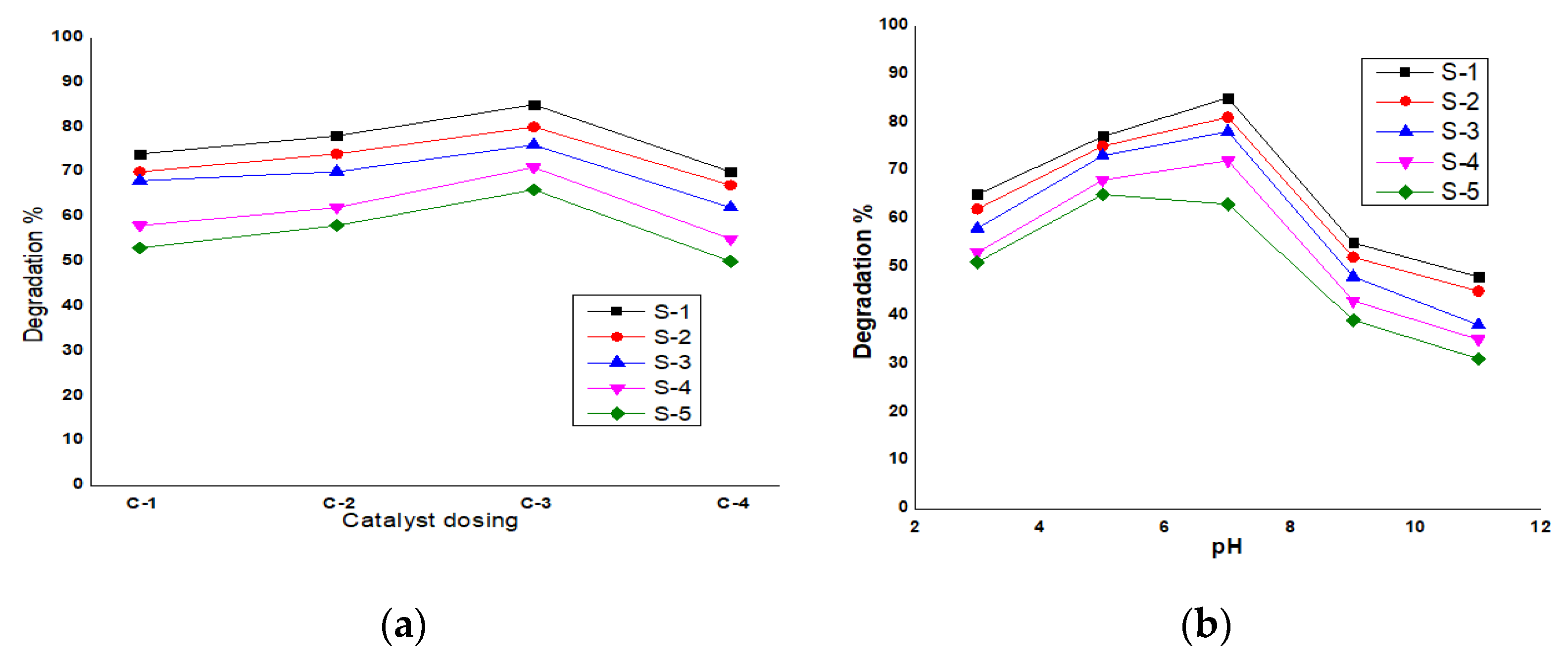
3.3. ZnO Degradation Efficiency at Different pH in Samples of Butachlor
3.4. ZnO Catalyst Degradation Efficiency at Different Doses
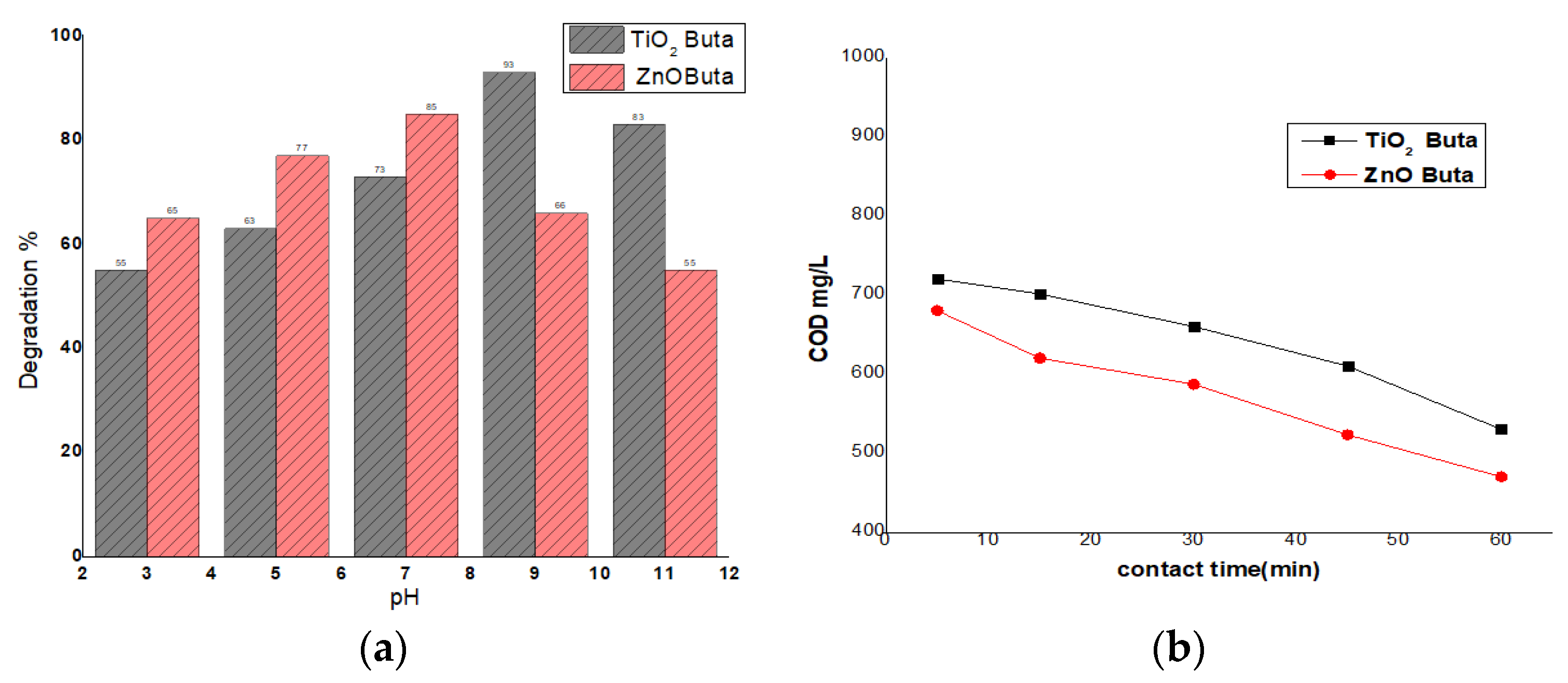
3.5. Comparison of Degradation Efficiency of ZnO and TiO2
3.6. Comparison of COD Values of ZnO and TiO2 over Time
4. Conclusions
Conflicts of Interest
References
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Abdollahi, Y.; Abdullah, A.H.; Zainal, Z.; Yusof, N.A. Photocatalytic degradation of p-Cresol by zinc oxide under UV irradiation. Int. J. Mol. Sci. 2012, 13, 302–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abigail, M.; Samuel, S.M.; Ramalingam, C. Addressing the environmental impacts of butachlor and the available remediation strategies: A systematic review. Int. J. Environ. Sci. Technol. 2015, 12, 4025–4036. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, M.; Ashoornia, M.; Panahandeh, M. Removal of Butachlor Toxin Polluted Water Using Ozonation, A Case Study of Groundwater Resources in Guilan Province. J. Water Wastewater 2015, 26, 114–123. [Google Scholar]
- Nozhat, S.; Fazilati, M.; Hassani, A.H. Butachlor and Diazinon Elimination from Aqueous Solution using TiO2/ZnO nano-photocatalysts. In Proceedings of the International Congress on Engineering Science and Sustainable Urban Development, Copenhagen, Denmark, 4 September 2018. [Google Scholar]
- Premalatha, N.; Miranda, L.R. Surfactant modified ZnO–Bi2O3 nanocomposite for degradation of lambda-cyhalothrin pesticide in visible light: A study of reaction kinetics and intermediates. J. Environ. Manag. 2019, 246, 259–266. [Google Scholar] [CrossRef]
- Darbandi, Z.; Zazouli, M.A.; Shokrzadeh, M.; Mousavinasab, N.; Ehsan, R. Photocatalytic degradation of diazinon using Zno/TiO2 nano-photocatalysts. KOOMESH 2016, 18, 343–349. [Google Scholar]
- Jonidi-Jafari, A.; Shirzad-Siboni, M.; Yang, J.; Naimi-Joubani, M.; Farrokhi, M. Photocatalytic degradation of diazinon with illuminated ZnO-TiO2 composite. J. Taiwan Inst. Chem. Eng. 2015, 50, 100–107. [Google Scholar] [CrossRef]
- Sraw, A.; Kaur, T.; Pandey, Y.; Sobti, A.; Wanchoo, R.K.; Toor, A.P. Fixed bed recirculation type photocatalytic reactor with TiO2 immobilized clay beads for the degradation of pesticide polluted water. J. Environ. Chem. Eng. 2018, 6, 7035–7043. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.; Abbas, G.; Tanveer, M.; Zubair, M. ZnO and TiO2 Assisted Photocatalytic Degradation of Butachlor in Aqueous Solution under Visible Light . Eng. Proc. 2021, 12, 77. https://doi.org/10.3390/engproc2021012077
Ahmad M, Abbas G, Tanveer M, Zubair M. ZnO and TiO2 Assisted Photocatalytic Degradation of Butachlor in Aqueous Solution under Visible Light . Engineering Proceedings. 2021; 12(1):77. https://doi.org/10.3390/engproc2021012077
Chicago/Turabian StyleAhmad, Mahmboob, Ghulam Abbas, Muhammad Tanveer, and Muhammad Zubair. 2021. "ZnO and TiO2 Assisted Photocatalytic Degradation of Butachlor in Aqueous Solution under Visible Light " Engineering Proceedings 12, no. 1: 77. https://doi.org/10.3390/engproc2021012077
APA StyleAhmad, M., Abbas, G., Tanveer, M., & Zubair, M. (2021). ZnO and TiO2 Assisted Photocatalytic Degradation of Butachlor in Aqueous Solution under Visible Light . Engineering Proceedings, 12(1), 77. https://doi.org/10.3390/engproc2021012077






