Degradation of Diclofenac under Irradiation of UV Lamp and Solar Light Using ZnO Photo Catalyst †
Abstract
:1. Introduction
2. Methodology
2.1. Material and Reagents
2.2. Experimental Analyzing Techniques
3. Result and Discussion
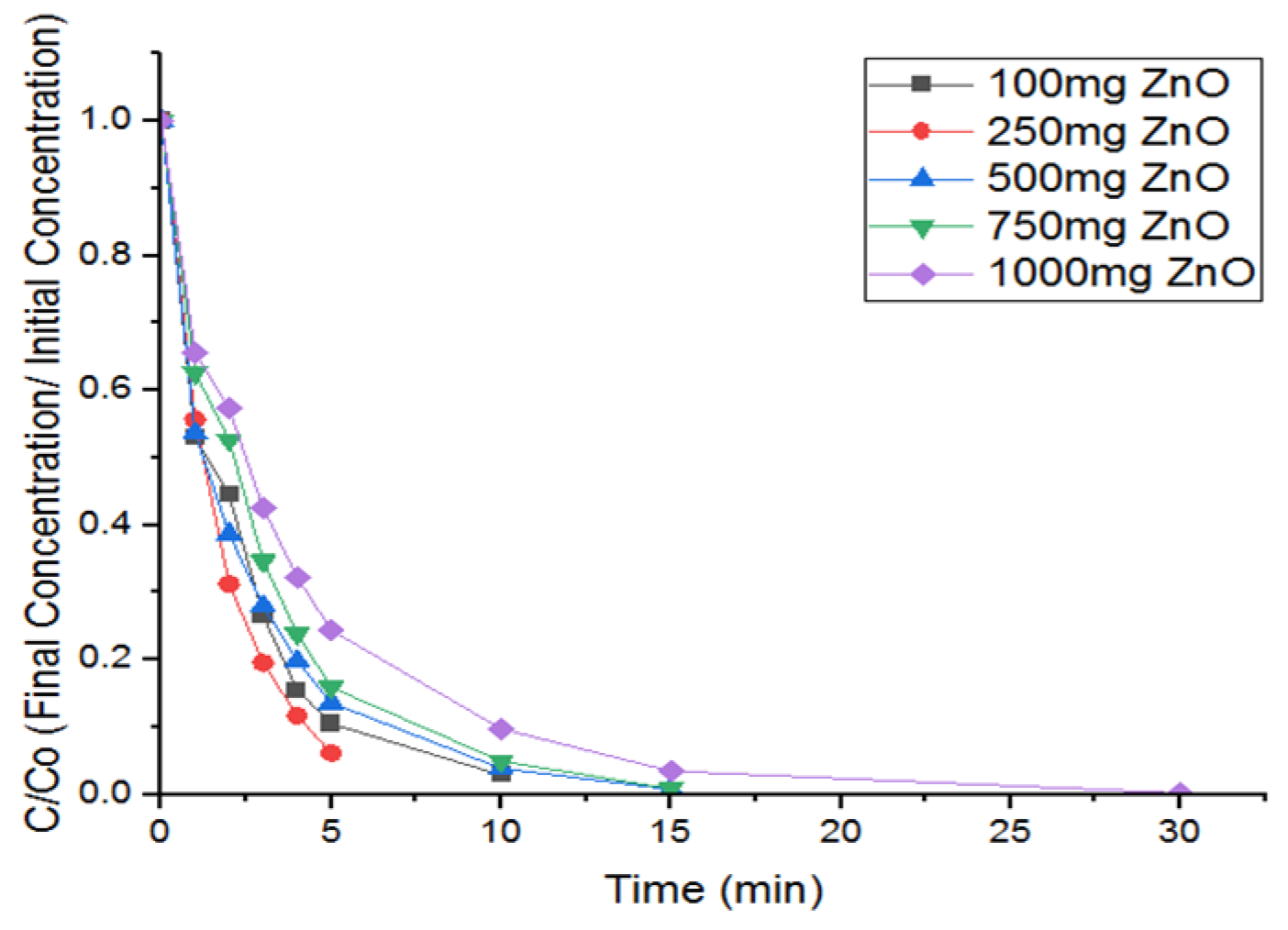
3.1. Effect of Catalyst Loading of ZnO
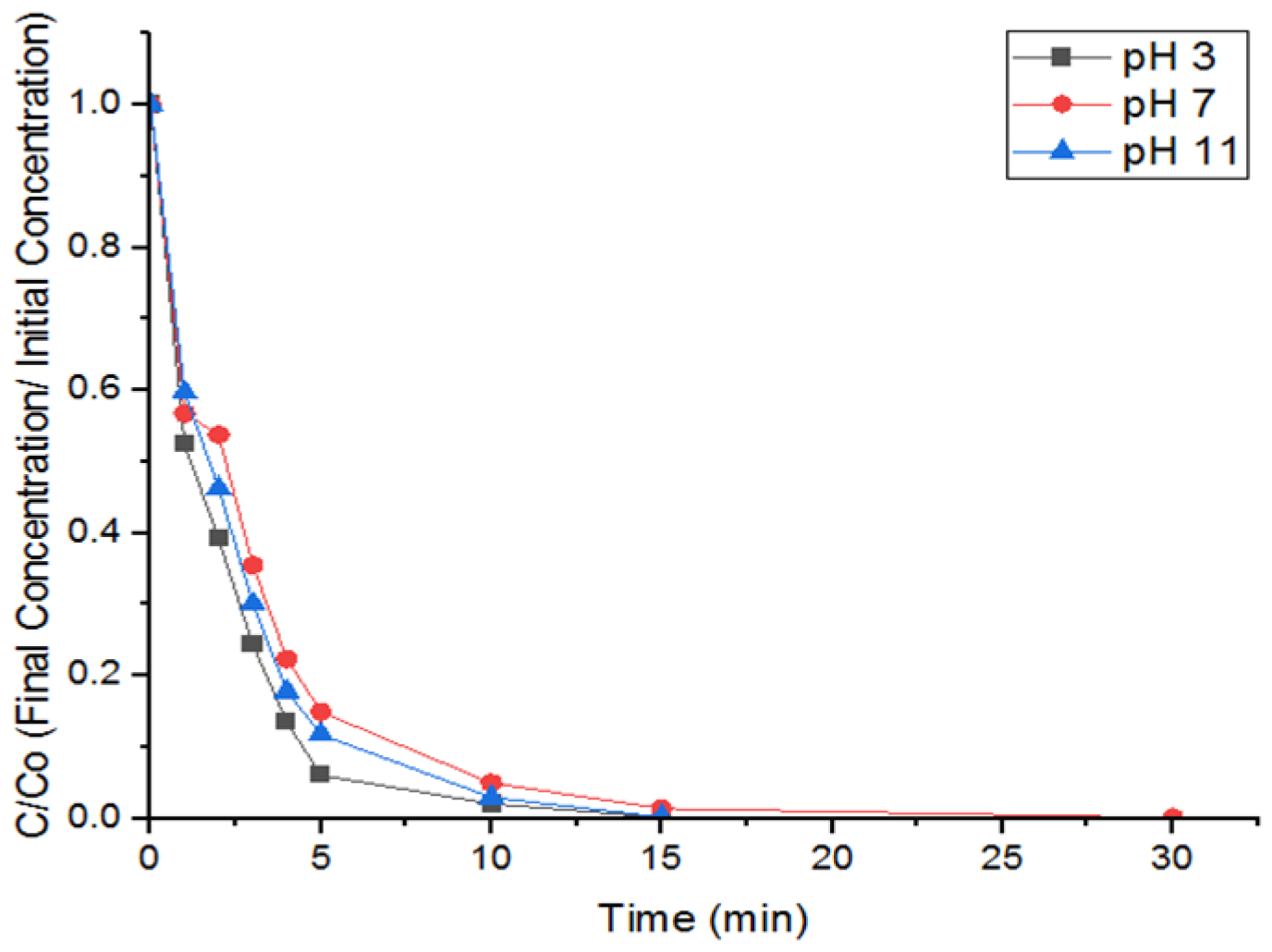
3.2. Effect of pH
3.3. Effect of Irradiation Source
4. Conclusions
Conflicts of Interest
References
- Bai, X.; Wang, X.; Lu, X.; Liang, Y.; Li, J.; Wu, L.; Li, H.; Hao, Q.; Ni, B.J.; Wang, C. Surface defective g-C3N4-xClx with unique spongy structure by polarization effect for enhanced photocatalytic removal of organic pollutants. J. Hazard. Mater. 2020, 398, 122897. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, N.; Zhu, R.; Li, S.; Yu, C.; Xia, W.; Xu, S.; Chen, X. Temperature-Program Assisted Synthesis of Novel Z-Scheme CuBi2O4/beta-Bi2O3 Composite with Enhanced Visible Light Photocatalytic Performance. Nanomaterials 2018, 8, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanveer, M.; Guyer, G.T.; Abbas, G. Photocatalytic degradation of Ibuprofen in water using TiO2 and ZnO under artifical UV and solar irradiation. Water Environ. Res. 2019, 91, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, B.; Wang, F.; Yuan, R.; Chen, H.; Han, X. Effect of dissolved organic matters and inorganic ions on TiO2 photocatalysis of diclofenac: Mechanistic study and degradation pathways. Environ. Sci. Pollut. Res. Int. 2020, 27, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, J.; Jiang, F.; Liu, P.; Zhu, M. Photo-assisted peroxymonosulfate activation via 2D/2D heterostructure of Ti3C2/g-C3N4 for degradation of diclofenac. Chemosphere 2020, 258, 127339. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Shahzad, A.; Woo, S.H.; Lee, D.S. Magnetic Ti3C2Tx (Mxene) for diclofenac degradation via the ultraviolet/chlorine advanced oxidation process. Environ. Res. 2020, 182, 108990. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, M.; Katic, J.; Kusic, H.; Loncaric Bozic, A.; Metikos Hukovic, M. Elucidating the Photocatalytic Behavior of TiO2-SnS2 Composites Based on Their Energy Band Structure. Materials 2018, 11, 1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Process | Degradation Rate (min−1) | TOC Reduction | COD Reduction |
|---|---|---|---|
| UV/ZnO | 0.403 | 51% | 83% |
| Solar (Borosilicate)/ZnO | 0.036 | 17% | 43% |
| Solar (Quartz)/ZnO | 0.015 | 15% | 47% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanveer, M.; Tezcanli, G.; Sadiq, M.T.; Kazmi, S.M.; Noshad, N.; Abbas, G.; Ali, A. Degradation of Diclofenac under Irradiation of UV Lamp and Solar Light Using ZnO Photo Catalyst. Eng. Proc. 2021, 12, 76. https://doi.org/10.3390/engproc2021012076
Tanveer M, Tezcanli G, Sadiq MT, Kazmi SM, Noshad N, Abbas G, Ali A. Degradation of Diclofenac under Irradiation of UV Lamp and Solar Light Using ZnO Photo Catalyst. Engineering Proceedings. 2021; 12(1):76. https://doi.org/10.3390/engproc2021012076
Chicago/Turabian StyleTanveer, Muhammad, Gokce Tezcanli, Muhammad Tahseen Sadiq, Syeda Memoona Kazmi, Nawal Noshad, Ghulam Abbas, and Asad Ali. 2021. "Degradation of Diclofenac under Irradiation of UV Lamp and Solar Light Using ZnO Photo Catalyst" Engineering Proceedings 12, no. 1: 76. https://doi.org/10.3390/engproc2021012076
APA StyleTanveer, M., Tezcanli, G., Sadiq, M. T., Kazmi, S. M., Noshad, N., Abbas, G., & Ali, A. (2021). Degradation of Diclofenac under Irradiation of UV Lamp and Solar Light Using ZnO Photo Catalyst. Engineering Proceedings, 12(1), 76. https://doi.org/10.3390/engproc2021012076





