An Open Source Water Quality Measurement System for Remote Areas †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tzortzakis, G.; Katsiri, E.; Karavokiros, G.; Makropoulos, C.; Delis, A. Tethys: Sensor-based aquatic quality monitoring in waterways. In Proceedings of the 2016 17th IEEE International Conference on Mobile Data Management (MDM), Porto, Portugal, 13–16 June 2016. [Google Scholar]
- Cloete, N.A.; Malekian, R.; Nair, L. Design of smart sensors for real-time water quality monitoring. IEEE Access 2016, 4, 3975–3990. [Google Scholar] [CrossRef]
- Afshan, N.; Kazmi, M.; Khan, S.A.; Jafri, N. Bacteriological survey of drinking water in Karachi. In Proceedings of the 2014 11th International Bhurban Conference on Applied Sciences & Technology (IBCAST), Islamabad, Pakistan, 14–18 January 2014. [Google Scholar]
- Guan, L.-L.; Li, Q.-S.; Jin, B.-E. Research of the Healthy Drinkable Water–Bama Recreate Water. In Proceedings of the 2012 International Conference on Biomedical Engineering and Biotechnology, Macau, Macao, 28–30 May 2012. [Google Scholar]
- Reddy, P.R.; Swaroop, G.S.; Teja, M.K.R. Mathematical analysis for constant household monitor of water pollution. In Proceedings of the 2010 IEEE International Conference on Electro/Information Technology, Normal, IL, USA, 20–22 May 2010. [Google Scholar]
- Suthar, S.; Carpenter, N.; Chhatralia, M. Smart Water Hardness Monitoring System. In Information and Communication Technology for Intelligent Systems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 595–601. [Google Scholar]
- Indu, K.; Choondal, J.J. Modeling, development & analysis of low cost device for water quality testing. In Proceedings of the 2016 IEEE Annual India Conference (INDICON), Bangalore, India, 16–18 December 2016. [Google Scholar]
- Haydar, S.; Arshad, M.; Aziz, J. Evaluation of drinking water quality in urban areas of Pakistan: A case study of Southern Lahore. Pak. J. Eng. Appl. Sci. 2016, 5, 16–23. [Google Scholar]
- Mounce, S.; Mounce, R.; Boxall, J. Case-based reasoning to support decision making for managing drinking water quality events in distribution systems. Urban Water J. 2016, 13, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Qamar, A.M. Data analysis, quality indexing and prediction of water quality for the management of rawal watershed in Pakistan. In Proceedings of the Eighth International Conference on Digital Information Management (ICDIM 2013), Islamabad, Pakistan, 10–12 September 2013. [Google Scholar]
- Peter, S.; Sreedevi, C. Water Quality Assessment and GIS mapping of ground water around KMML industrial area, Chavara. In Proceedings of the 2012 International Conference on Green Technologies (ICGT), Trivandrum, India, 18–20 December 2012. [Google Scholar]
- Barron, J.J.; Ashton, C. The effect of temperature on conductivity measurement. TSP 2005, 7, 1–5. [Google Scholar]
- Faruq, M.O.; Emu, I.H.; Haque, M.N.; Dey, M.; Das, N.K.; Dey, M. Design and implementation of cost effective water quality evaluation system. In Proceedings of the 2017 IEEE Region 10 Humanitarian Technology Conference (R10-HTC), Dhaka, Bangladesh, 21–23 December 2017. [Google Scholar]
- Yuwono, T.; Pramono, W.B.; Ardi, I.; Hakim, L.; Ismail, M. Design of the remote sensing circuit for water conductivity. In Proceedings of the 2015 International Conference on Space Science and Communication (IconSpace), Langkawi, Malaysia, 10–12 August 2015. [Google Scholar]

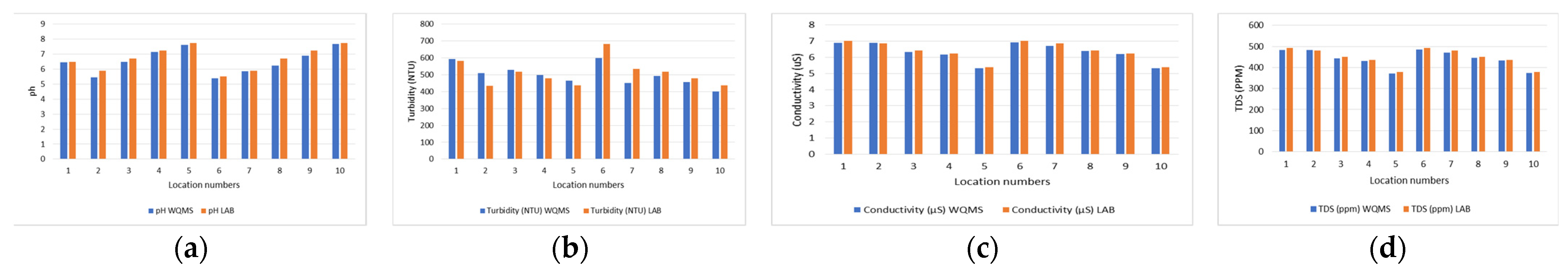
| Sample No # | pH [10] | Turbidity (NTU) [7] | Conductivity (µS) [7] | TDS (ppm) [7] |
|---|---|---|---|---|
| Admissible Range | 6.5 to 8.0 | 0 to 500 | 0 to 5 | 0 to 500 |
| Sample from Location No # | pH WQMS | pH LAB | Turbidity (NTU) WQMS | Turbidity (NTU) LAB | Conductivity (µS) WQMS | Conductivity (µS) LAB | TDS (ppm) WQMS | TDS (ppm) LAB |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.45 | 6.50 | 593 | 583 | 6.90 | 7.03 | 483 | 492 |
| 2 | 5.45 | 5.90 | 509 | 435 | 6.9 | 6.87 | 483 | 481 |
| 3 | 6.48 | 6.70 | 528 | 517 | 6.33 | 6.43 | 443.1 | 450 |
| 4 | 7.14 | 7.23 | 499 | 478 | 6.17 | 6.25 | 431.9 | 437 |
| 5 | 7.62 | 7.75 | 464 | 437 | 5.32 | 5.40 | 372.4 | 378 |
| 6 | 5.38 | 5.50 | 598 | 683 | 6.92 | 7.03 | 484.4 | 492 |
| 7 | 5.87 | 5.90 | 450 | 535 | 6.72 | 6.87 | 470.4 | 481 |
| 8 | 6.23 | 6.70 | 492 | 517 | 6.38 | 6.43 | 446.6 | 450 |
| 9 | 6.90 | 7.23 | 458 | 478 | 6.20 | 6.25 | 434 | 437 |
| 10 | 7.68 | 7.75 | 401 | 437 | 5.33 | 5.40 | 373.1 | 378 |
| Sample No # | LAB | WQMS | Quality Decision | |||
|---|---|---|---|---|---|---|
| In Range | Out of Range | In Range | Out of Range | In Range | Out of Range | |
| 1 | 2 | 2 | 2 | 2 | Neutral | Neutral |
| 2 | 2 | 2 | 2 | 2 | Neutral | Neutral |
| 3 | 2 | 2 | 1 | 3 | Neutral | Undrinkable |
| 4 | 2 | 2 | 3 | 1 | Neutral | Good |
| 5 | 3 | 1 | 3 | 1 | Good | Good |
| 6 | 1 | 3 | 1 | 3 | Undrinkable | Undrinkable |
| 7 | 1 | 3 | 2 | 2 | Undrinkable | Neutral |
| 8 | 2 | 2 | 2 | 2 | Neutral | Neutral |
| 9 | 3 | 1 | 3 | 1 | Good | Good |
| 10 | 3 | 1 | 3 | 1 | Good | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, M.O.; Siddiq, A.; Irshad, H.; Aman, M.; Khan, M.S. An Open Source Water Quality Measurement System for Remote Areas. Eng. Proc. 2021, 12, 50. https://doi.org/10.3390/engproc2021012050
Tariq MO, Siddiq A, Irshad H, Aman M, Khan MS. An Open Source Water Quality Measurement System for Remote Areas. Engineering Proceedings. 2021; 12(1):50. https://doi.org/10.3390/engproc2021012050
Chicago/Turabian StyleTariq, Muhammad Owais, Asif Siddiq, Hafsa Irshad, Muhammad Aman, and Muhammad Shahbaz Khan. 2021. "An Open Source Water Quality Measurement System for Remote Areas" Engineering Proceedings 12, no. 1: 50. https://doi.org/10.3390/engproc2021012050
APA StyleTariq, M. O., Siddiq, A., Irshad, H., Aman, M., & Khan, M. S. (2021). An Open Source Water Quality Measurement System for Remote Areas. Engineering Proceedings, 12(1), 50. https://doi.org/10.3390/engproc2021012050







