1. Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune endocrine disorder characterized by the progressive destruction of pancreatic beta cells, which are responsible for insulin production. The direct consequence for patients is total dependence on daily exogenous insulin therapy, as well as the need for continuous monitoring of blood glucose levels. This ongoing monitoring represents a considerable burden for people with T1DM, as it involves constant attention, multiple decision making throughout the day, and close surveillance to avoid both acute (hypoglycemia and hyperglycemia) and chronic (retinopathy, nephropathy, neuropathy, among others) complications.
For decades, treatment of T1DM has been based on subcutaneous insulin administration via multiple daily injections or continuous infusion pumps and glycemic control via capillary punctures. However, this traditional approach has several limitations. The main one is that blood sugar measurements are intermittent, making it difficult to interpret glycemic variability in real time. The therapeutic decisions (doses administered, early detection of hypoglycemia and hyperglycemia) fall on the patient. This constant and autonomous management of the disease is associated with fatigue and emotional burden, leading in some cases to demotivation or irregular adherence to treatment.
In this context, the development of portable technologies for continuous glucose monitoring (WGS) has been a significant advance. These devices adhere to the patient’s skin, usually on the arm, and continuously measure glucose levels in the interstitial fluid, offering a much more precise and dynamic view of glycemic changes. This continuous measurement, using artificial intelligence, allows for anticipating risk situations, adjusting insulin doses based on trends, and reducing the frequency of fingerstick, which positively impacts the patient’s quality of life. The impact of these sensors has been even greater when integrated with automated insulin delivery systems, in what are known as “artificial pancreas” or hybrid closed-loop systems. This technology combines three components: a WGS sensor, an insulin pump, and an algorithm that automatically calculates and adjusts insulin doses based on detected glucose levels and other relevant factors (e.g., heart rate, galvanic skin response, body temperature, or physical activity level). This integration allows for a more holistic assessment of a person’s metabolic status and contributes to improving the predictive capacity of control algorithms. Thanks to these systems, it is possible to maintain glucose values within safer ranges without constant manual intervention, which reduces both the physical and mental burden of treatment.
However, the incorporation of these technologies into the daily treatment of T1DM does not depend solely on their technical performance. For them to have a real impact on people’s health and well-being, they must be accepted, understood, and used correctly by those living with this disease. In this sense, patient acceptance becomes a determining factor for the success of any innovation in digital health. For this reason, in addition to studying the clinical efficacy of wearable sensors and automated systems, it is essential to analyze how they are perceived by people with T1DM. Understanding their direct experience, gathering their opinions, and understanding their expectations allows us to better guide the design of these technologies and facilitate their adoption in real-life practice.
The objective of this paper is, therefore, to present a detailed analysis of current innovations in wearable glucose sensors and their integration with smart insulin delivery systems, based on available clinical evidence. It also explores the levels of acceptance observed among people with T1DM, using both bibliographic sources and testimonials collected in different settings. The aim is to offer a broad and well-founded overview of the impact of these technologies on personalized disease management, as well as the challenges that remain to be addressed for effective and sustainable implementation.
2. Materials and Methods
This work was carried out through a literature review to understand the current status of portable technologies applied to the personalized management of T1DM, specifically glucose sensors and automated insulin delivery systems. Various recognized scientific platforms were used to collect the information, including Scopus, Google Scholar, and Wiley Online Library. These search engines were selected for their multidisciplinary coverage and the quality of the indexed publications. The search was conducted using combinations of key terms such as “wearable glucose monitoring”, “non-invasive sensors”, “artificial pancreas”, “type 1 diabetes technologies” and “patient acceptance”. Inclusion criteria were applied to limit the search to works published since 2017, with the aim of focusing on recent technologies and studies that reflect the current context. Articles that provided clinical evidence, technical data, and user experiences in the use of these technologies were selected, excluding outdated publications or those focused on early development phases.
In addition to the technical and clinical analysis, studies specifically addressing the degree of acceptance by people with T1DM were reviewed. Previous research using structured questionnaires as a data collection tool was consulted, focusing on aspects such as the ease of use of the devices, perception of glycemic control, feeling of safety when using them, and intention to continue using them long-term. Qualitative sources were also considered, including the direct experience of people with T1DM who have already used these technologies in their daily lives. These testimonies offered additional information on the comfort of daily use, the usefulness of the automated functions, and the main difficulties encountered in the first weeks, especially during the adaptation and calibration phase of the system.
This methodological approach allowed us to combine quantitative and qualitative evidence, providing a more complete view of both the technical effectiveness of the technologies studied and their acceptance among patients. The triangulation of sources provided robustness to the analysis and allowed us to identify both the strengths and challenges that persist in the clinical implementation of these tools.
3. Results and Discussion
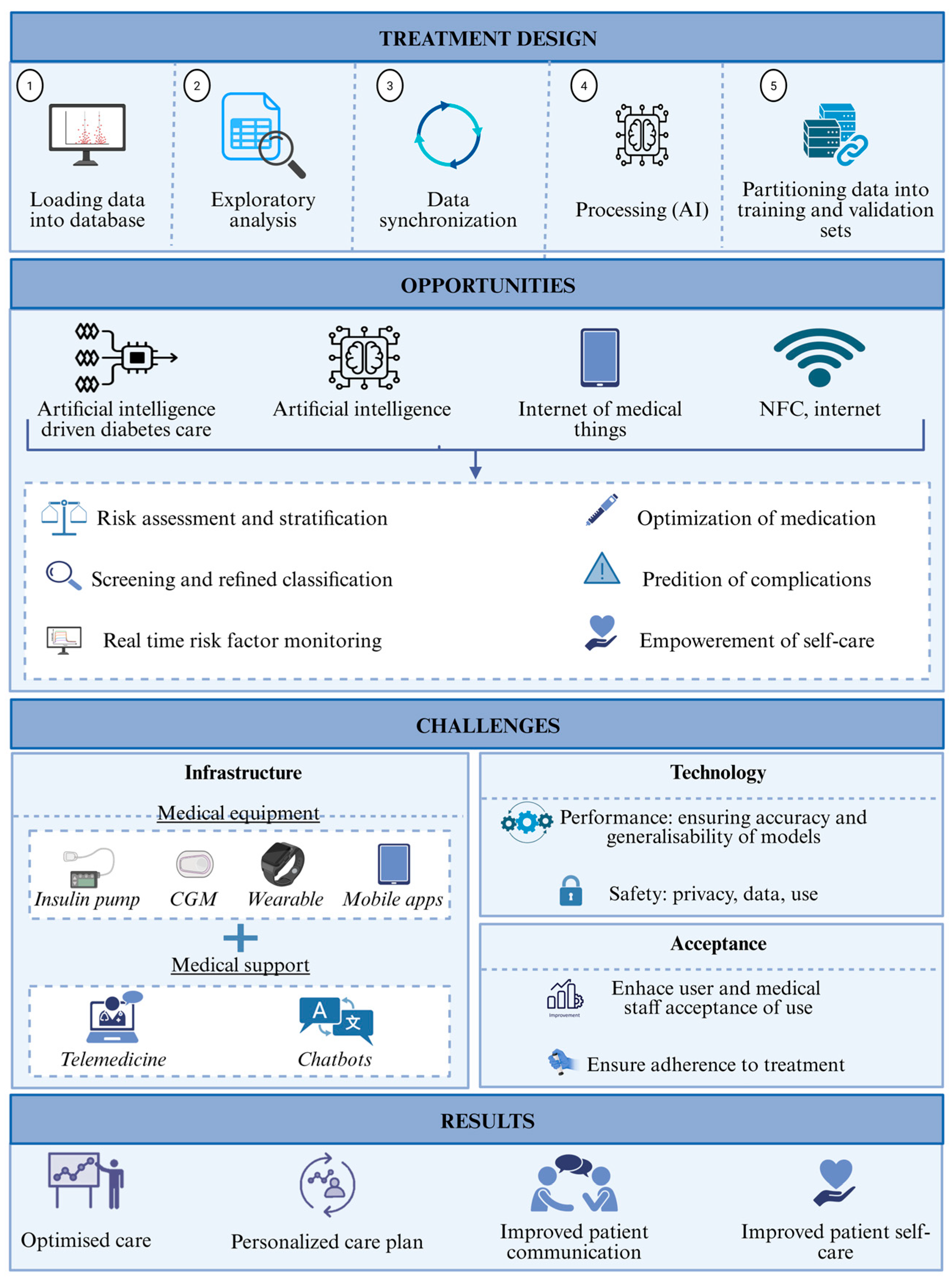
Continuous glucose monitoring has evolved beyond its initial passive monitoring function (
Figure 1). Today, sensors can provide highly accurate, real-time readings, and the data is processed using algorithms that detect trends and prevent extreme events, such as nocturnal hypoglycemia or postprandial spikes. Recent advances focus on achieving these same capabilities using less invasive equipment. Newly proposed systems include devices that measure glucose through the skin using light at different wavelengths and equipment that analyzes sweat components to identify correlations with blood glucose. Although these methods have not yet achieved the reliability of traditional systems, their less intrusive nature makes them an attractive alternative from the patient’s perspective.
Devices that combine glucose sensors with insulin pumps and control algorithms are bringing the possibility of near-autonomous diabetes management closer. These systems adjust doses in real time, based on each person’s metabolic needs, thus reducing the burden of daily treatment decisions. The clinical efficacy of these solutions has been confirmed in various studies, with observed improvements in time in range, lower glycemic variability, and a reduced risk of complications. However, they are not without challenges. Aspects such as the delay in detecting changes, the difficulty in adapting to specific physiological situations (such as intense physical exercise), and the lack of compatibility between devices from different manufacturers continue to limit their widespread use.
One of the key factors for the success of these technologies is their acceptance by the people who use them. In general, there is a favorable disposition to incorporate them into daily routines, especially when improvements in quality of life are perceived. However, some initial reluctance is also identified. During the first few weeks, many users experience an adaptation phase during which they must familiarize themselves with the system’s functions and trust that the automated decisions are correct. A common problem during this period is excessive notifications or alerts, which can generate a feeling of fatigue or stress, particularly in people already mentally overloaded by daily disease management. Over time, these barriers tend to decrease, especially if the system is tailored to individual needs and the patient feels supported throughout the process.
Figure 1.
Advancements in diabetes care equipment.
Figure 1.
Advancements in diabetes care equipment.
Clinical evidence shows that the combined use of wearable sensors and control algorithms significantly improves the management of type 1 diabetes. People who use these systems manage to maintain their glucose levels within the recommended range for longer and experience fewer episodes of severe hypoglycemia. A decreased need for manual interventions and greater stability in glycemic control have also been observed. These benefits contribute not only to reducing the risk of long-term complications but also to improving patients’ perceived quality of life.
Regarding the user experience, the first few days often require an adjustment period during which the algorithm learns to respond more accurately to individual patterns. Although this process can generate some uncertainty, most users adapt quickly and report a high level of satisfaction once this stage is over. The testimonials collected indicate that the combination of technology, education, and professional support is essential for the system to function effectively. Similarly, the customization of alerts and the ability to modify sensitivity parameters allow for a more natural integration into everyday life.
4. Conclusions
Current technologies for managing T1DM are advancing toward more automated, efficient, and personalized care. Wearable glucose sensors, along with smart insulin delivery systems, can improve both clinical outcomes and the quality of life of those who use them.
Acceptance among people with T1DM is generally high, especially when tools are offered that meet their real needs and their adaptation times are respected. Trust in the system, clear information received, and the ability to make shared decisions with healthcare professionals are key elements for successful adoption.
As these technologies continue to develop, it will be essential to address not only the technical challenges but also the ethical and human aspects involved in their use. Listening to the voices of patients and facilitating their active participation in the innovation process will continue to be an essential component for moving toward more effective and sustainable management of T1DM.