1. Introduction
Volatile organic compounds (VOCs) are increasingly of concern in environmental, industrial, and health applications [
1]. Among these, acetone stands out as a noninvasive diabetes biomarker [
2]. Despite the high precision of analytical techniques like gas chromatography–mass spectrometry to monitor VOCs, they are expensive, time-consuming, and not portable. This has propelled the need for small, cheap, and very selective chemoresistive gas sensors [
3].
Nanostructured metal oxides (MOXs) such as SnO
2, WO
3, In
2O
3, and Fe
2O
3 have been extensively investigated as functional materials for solid-state gas sensor development. Nevertheless, there is still a persistent demand in the market for chemoresistive gas sensors that offer enhanced material control to achieve better gas sensitivity, improved long-term stability, and more precise detection capabilities under varying environmental conditions across diverse application scenarios. The results are particularly significant with regard to solid solution mixtures of MOX materials [
4,
5,
6]. This strategy allows alteration of the structural and electronic properties of MOX lattices, to improve their gas sensitivity and selectivity. Rare-earth-based oxides have proved promising for improving gas sensitivity, although they are less explored than transition metals [
7,
8,
9]. Specifically, the compounds that contain praseodymium, including PrFeO
3 [
10], are highly sensitive to acetone, although they are prone to humidity interference. This effect can be minimized through the addition of titanium (Ti) to the oxide lattice, which restricts the development of surface hydroxyl groups, which compete with the adsorption of target gases [
11,
12]. Although the roles of these elements have been identified, the use of new Pr-based solid solutions for highly selective acetone detection has not been extensively studied. This paper fills this gap through the study of PrFeTiO
5 as a new sensing material that can undergo reliable and selective acetone detection.
2. Materials and Methods
Synthesis of PrFeTiO5
PrFeTiO
5 was synthesized by mixing, in stoichiometric proportions (1:1:1 molar ratio of Pr:Fe:Ti in the final compound), praseodymium oxide (Pr
6O
11), iron (III) oxide (Fe
2O
3), and titanium dioxide (TiO
2) by the solid-state reaction method. All chemicals were purchased from sigma-Aldrich (Casablanca/Morocco) In order to obtain a high-purity phase, the mixture was heated in an open-air muffle furnace up to 1200 °C over 24 h [
13].
Materials’ and Functional Films’ Characterization
The structural, morphological, and compositional characteristics of the samples were studied using scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), and X-ray powder diffraction (XRPD).
Investigations were conducted into the morphology and homogeneity of both powders and screen-printed sensing films using a Zeiss LEO 1530 FEG SEM (manufactured by Carl Zeiss, located in Oberkochen, Germany) along with an Oxford Instruments INCA 250 EDS system for performing elemental analysis. The PrFeTiO5 sample was prepared as a cross-sectional lamella through Ga-ion focused ion beam milling within a ZEISS Crossbeam 340 dual-beam FIB–SEM system and subsequently analyzed using a TECNAI F20 TEM at 200 kV in both HRTEM and STEM–EDS modes.
XRPD analyses were performed on a Bruker D8 Advance Da Vinci diffractometer (manufactured by Bruker, Karlsruhe, Germany), which employed CuKα radiation and a LynxEye XE silicon strip detector in a Bragg–Brentano geometry configuration. Samples were mounted on a zero-background holder with a knife-edge placed to decrease air scattering, and measurements were taken at room temperature over a 2θ range of 3–120°, with a step size of 0.02° and a counting time of 2 s per step. Phase identification was performed using EVA version 6.0, and Rietveld refinement was carried out with TOPAS version 5.0, as provided by Bruker.
Film deposition and sensor development
The synthesized powders were mixed with α-terpineol, ethyl cellulose, and silica to create uniform pastes for sensor development [
14]. In this formulation, α-terpineol (a mixture of isomers with a purity of more than 96% from Sigma-Aldrich (St. Louis, MO, USA) and a 5%
w/
w solution of ethyl cellulose in an 80:20 toluene/ethanol mixture (from Sigma-Aldrich, measured at 25 °C) served as the binders and solvent. The organic content was adjusted between 50 and 80% by weight, with silica added in the range of 0.5–1% by weight. The addition of silica improved adhesion within the nanostructured material and between the sensing layer and the alumina substrate.
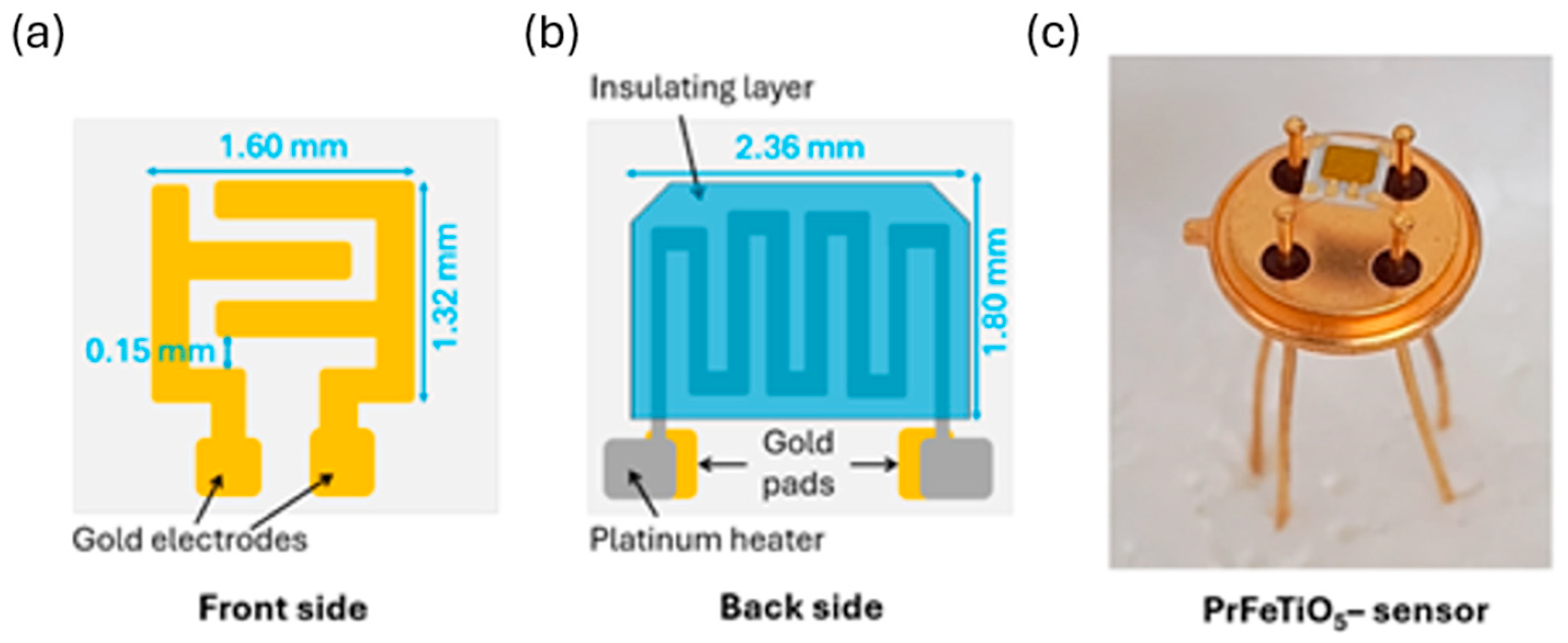
The paste was then deposited using an AUREL C920 screen printer onto the alumina substrate containing interdigited gold electrodes on the front surface for electrical measurements (
Figure 1a) and a platinum heater mounted on the back side for thermal activation (
Figure 1b). The printed films featured an active area of roughly 1 mm
2 and a thickness of 20–30 μm.
After the deposition process, the sensors were heat-treated at 650 °C in air for 2 h to enhance grain connectivity, ensure structural stability during operation, eliminate residual organic compounds, and increase the bond strength between the film and the substrate.
The device was packaged using thermo-compression wedge bonding of four contacts to the commercially available TO-39 support with 0.06 mm diameter gold wire. The packaged sensor was then integrated into the measurement system.
Gas Sensing Measurements
The sensing performance of the sensor was assessed with a specially designed setup that included a sealed gas test chamber with a volume of 622 cm
3 and a data acquisition system. A central gas diffuser was situated within the chamber, while a series of mass-flow controllers regulated the total gas flow at a constant rate of 500 sccm. Monitoring of temperature and relative humidity (RH%) was carried out using a commercially available LM35 temperature sensor and a Honeywell HIH-4000 humidity sensor. The chamber temperature was maintained constant at 25 °C within a climatic box to ensure consistent testing conditions. A constant bias voltage of 5 volts was applied across the interdigitated electrodes to characterize the sensor electrically. The output voltage (V
out) was measured across a series load resistor (R
f) using an operational amplifier-based circuit. The sensor’s resistance (R
s) was subsequently calculated as
and its corresponding sensor conductance (G
s) was obtained as its direct reciprocal.
The measured voltage signal is therefore directly proportional to the sensing layer’s conductance [
15]. Then, for an n-type MOX sensor, the response to a reducing gas is defined as
where G
gas and G
air are the conductance values in the presence of testing gas and synthetic air, respectively.
The experimental measurements were arranged as follows.
Operating temperature: The operating temperature is an important parameter that can be used to tune and optimize the sensing performance of MOX-based gas sensors, because it influences the surface reaction kinetics, charge transfer process, and overall performance of gas sensors. The response of the PrFeTiO5 sensor to 5 ppm acetone was tested at different working temperatures between 350 °C and 450 °C.
Sensitivity to acetone: The conductance variation of sensing film was studied with acetone concentrations of 5, 10, 25, and 50 ppm in both dry (2 RH %) and humid (17 RH%) conditions.
Calibration curves: The response vs. the acetone concentration was assessed in dry and wet (17 RH%) atmospheres.
Humidity effect: To study the influence of humidity on the sensor’s response, different mixtures of dry and wet air were used to adjust the relative humidity inside the test chamber over a range of 2–41 RH%. Throughout the measurements the acetone concentration was maintained at 10 ppm.
Selectivity: The selection of interferents was made to reflect the possible co-existence of gases in industry applications, while also covering a range of chemical functional groups to assess cross-sensitivity. The sensor’s selectivity was evaluated by exposing it to concentrations of 100 ppm H2, 25 ppm NH3, 1200 ppm CO2 and 25 ppm of CO, which were chosen in accordance with TLV-TWA guidelines and the relevant literature data on environmental gases.
3. Results and Discussion
Structural, Morphological, and Chemical Characterization
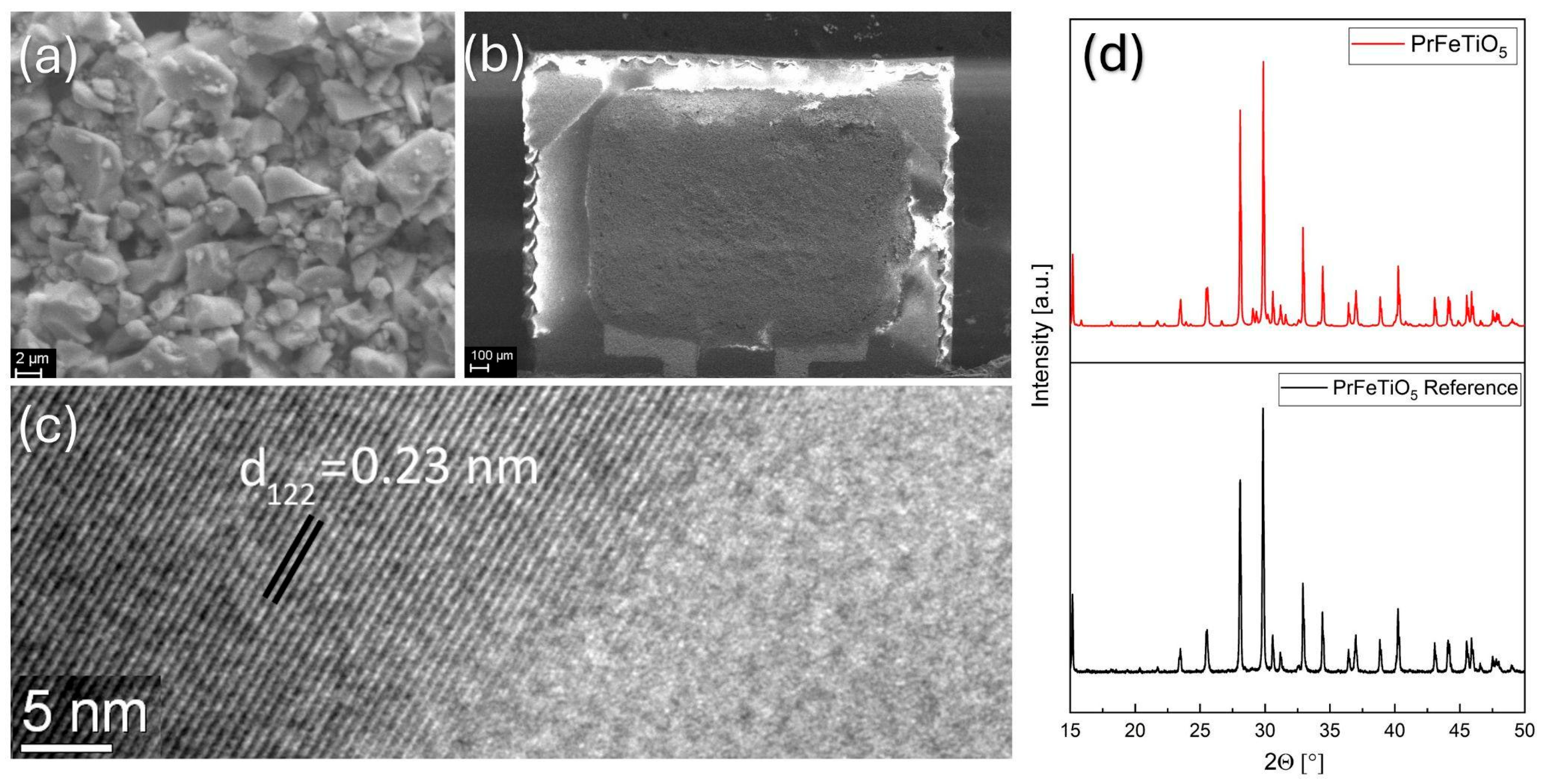
The morphology of a synthesized solid solution as seen by SEM is shown in
Figure 2a, where compact micrometer-sized grains emerge during coalescence at 1200 °C. As a result of the screen printing, the substrate was uniformly covered by the PrFeTiO
5 thick film (
Figure 2b).
Figure 2c shows HRTEM images of large single-crystal domains with distinct grain boundaries. The X-ray diffraction patterns in
Figure 2d confirm PrFeTiO
5 as the main crystalline phase (84.4 wt%) with significant crystallinity.
Gas Sensing Performance
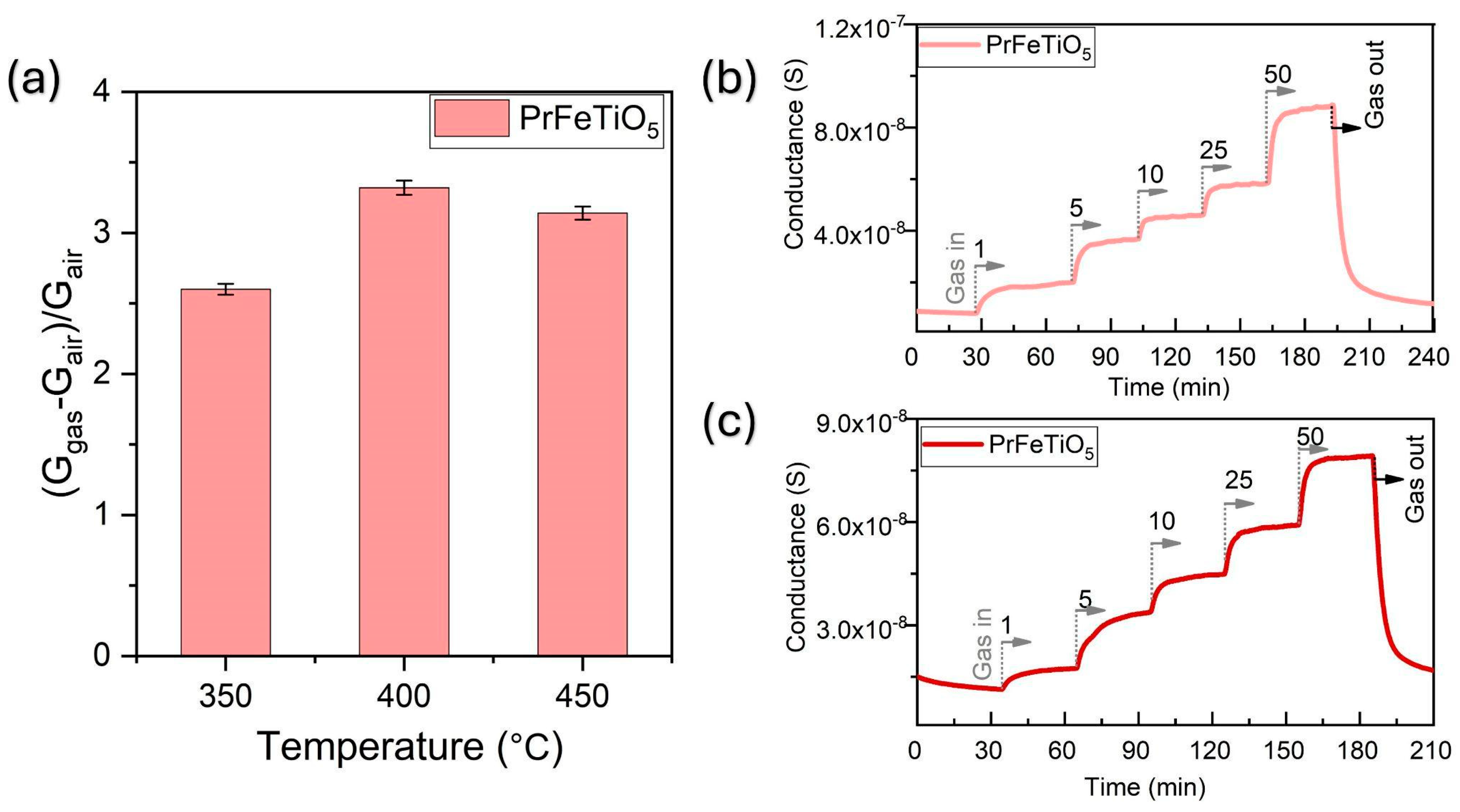
Operating temperature: The conductance of the functional film below 350 °C was too low to be measured using the available data acquisition system (
Figure 3a). The response increased with temperature, with a maximum at 400 °C, at which point surface reactivity and adsorption time were in balance. Above this point, reduced interaction time resulted in a decrease in response, confirming that 400 °C is the optimal operating temperature.
Sensitivity to acetone: The conductance of an n-type semiconductor increases when exposed to acetone, a reducing gas, due to its interaction with adsorbed oxygen, which releases electrons into the semiconductor’s conduction band.
Figure 3b,c show a progressive increase in conductance as acetone concentration rises from 5 to 50 ppm, with stabilization at each concentration level and full recovery after acetone is removed. This reversible and constant response occurs consistently in both dry (
Figure 3b) and humid (
Figure 3c) conditions, demonstrating the sensor’s reliable performance across varying humidity levels.
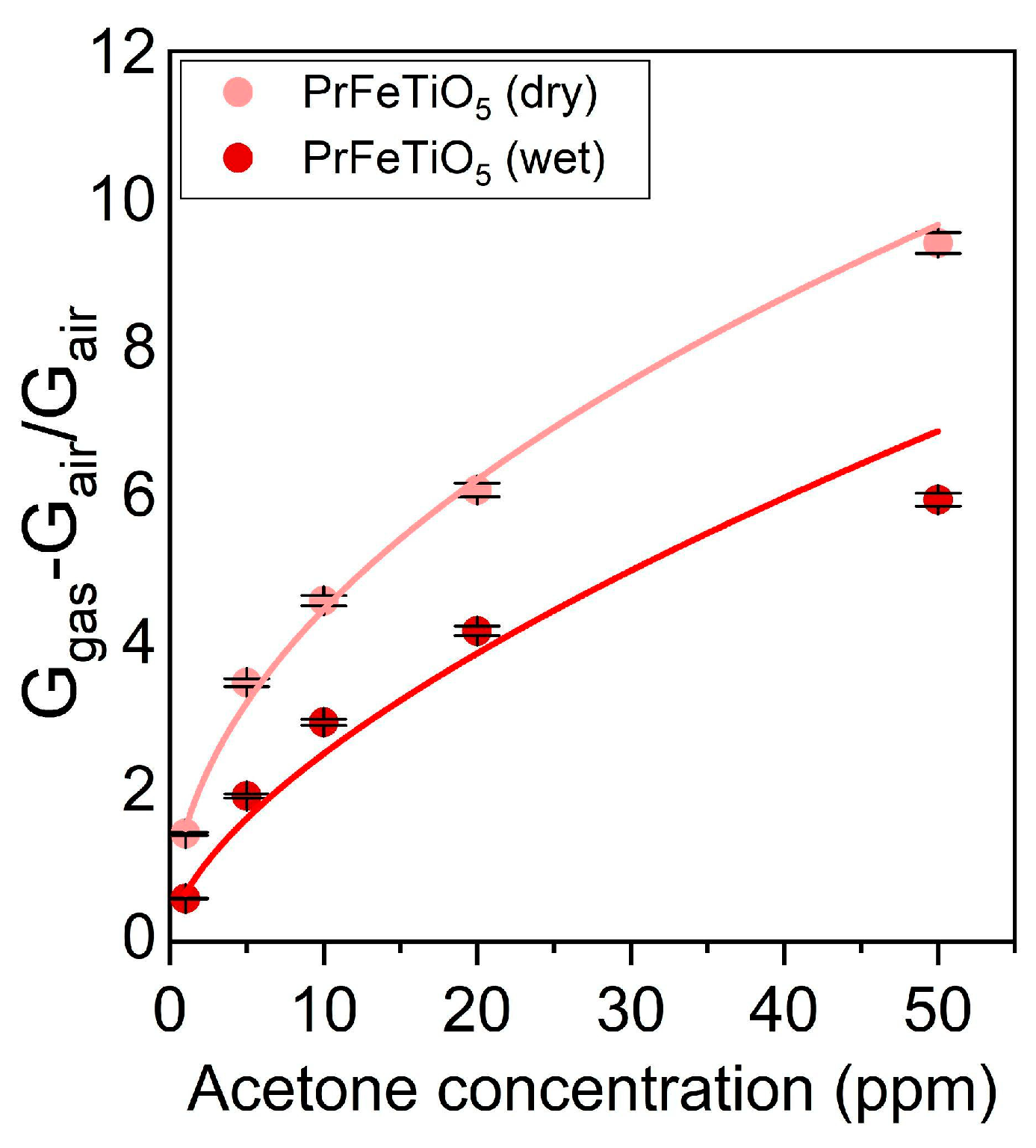
Calibration curves: The response level of the sensor for acetone 1–50 ppm increased nonlinearly under both dry and humid (17 RH) conditions. As shown in
Figure 4, the responses to concentration
x were fitted with a power law function
R =
axb, with
R being the sensor response. The parameters
a and
b were equal to 1.45 ± 0.07 and 0.484 ± 0.019 in dry conditions and 0.58 ± 0.08 and 0.63 ± 0.05 at 17 RH%. The presence of humidity reduced the response to the analyte due to water molecules competing with acetone on the sensor’s active sites, thereby limiting acetone interaction.
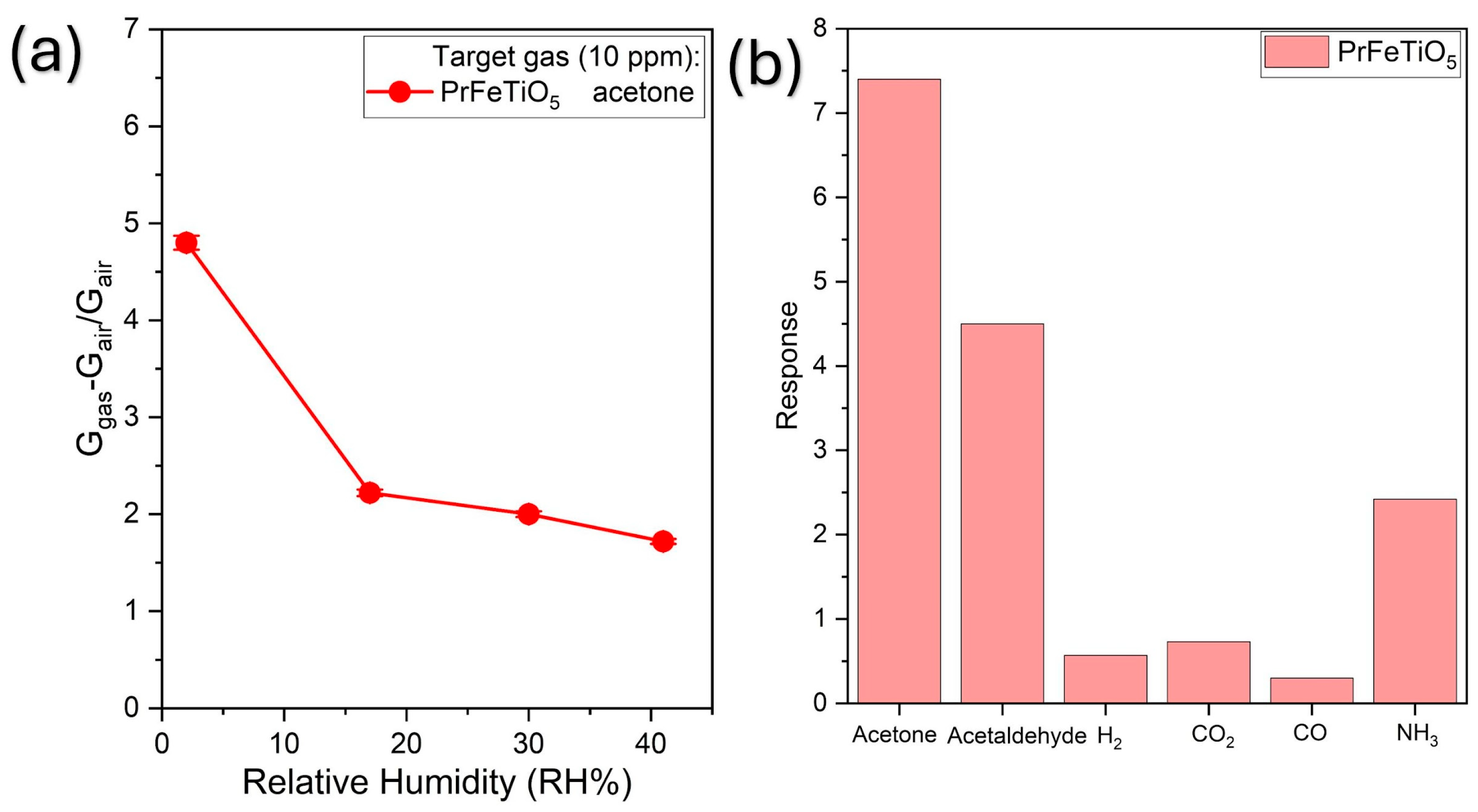
Humidity effect: The influence of humidity on the sensor’s response was further investigated by measuring 10 ppm acetone across a wide range of RH levels (2–41%).
Figure 5a illustrates that the sensor displayed a pronounced initial drop in response as the RH increased from 2% to 17%. The response then decreases more slowly at higher humidity levels, implying that the most active surface sites become quickly saturated with water molecules at low RH; subsequent water absorption has a decreasing impact on the overall signal.
Selectivity:
Figure 5b demonstrates the sensor’s pronounced selectivity towards acetone; indeed, its response is much higher than that of the other tested gases, i.e., acetaldehyde, NH
3, CO, CO
2, and H
2.