Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity †
Abstract
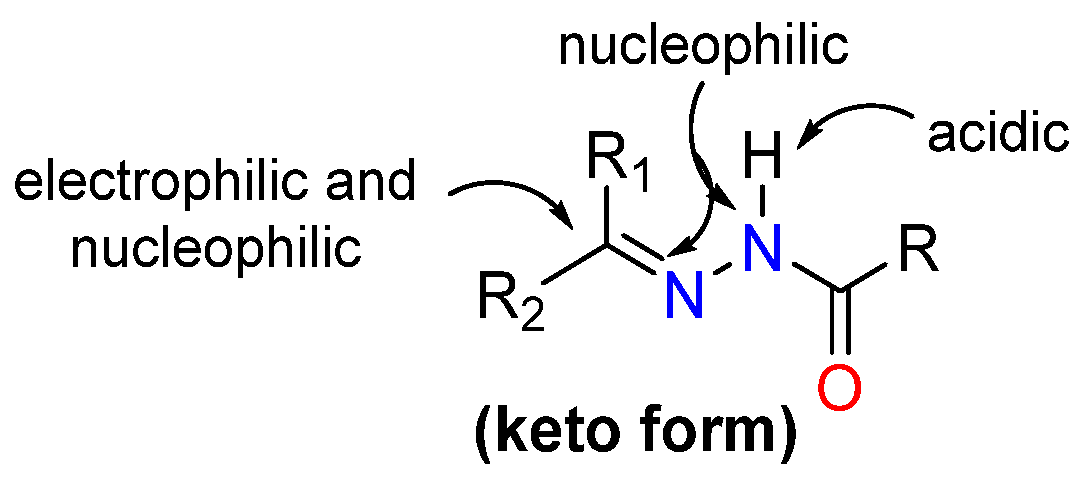
1. Introduction
2. Medicinal Chemistry
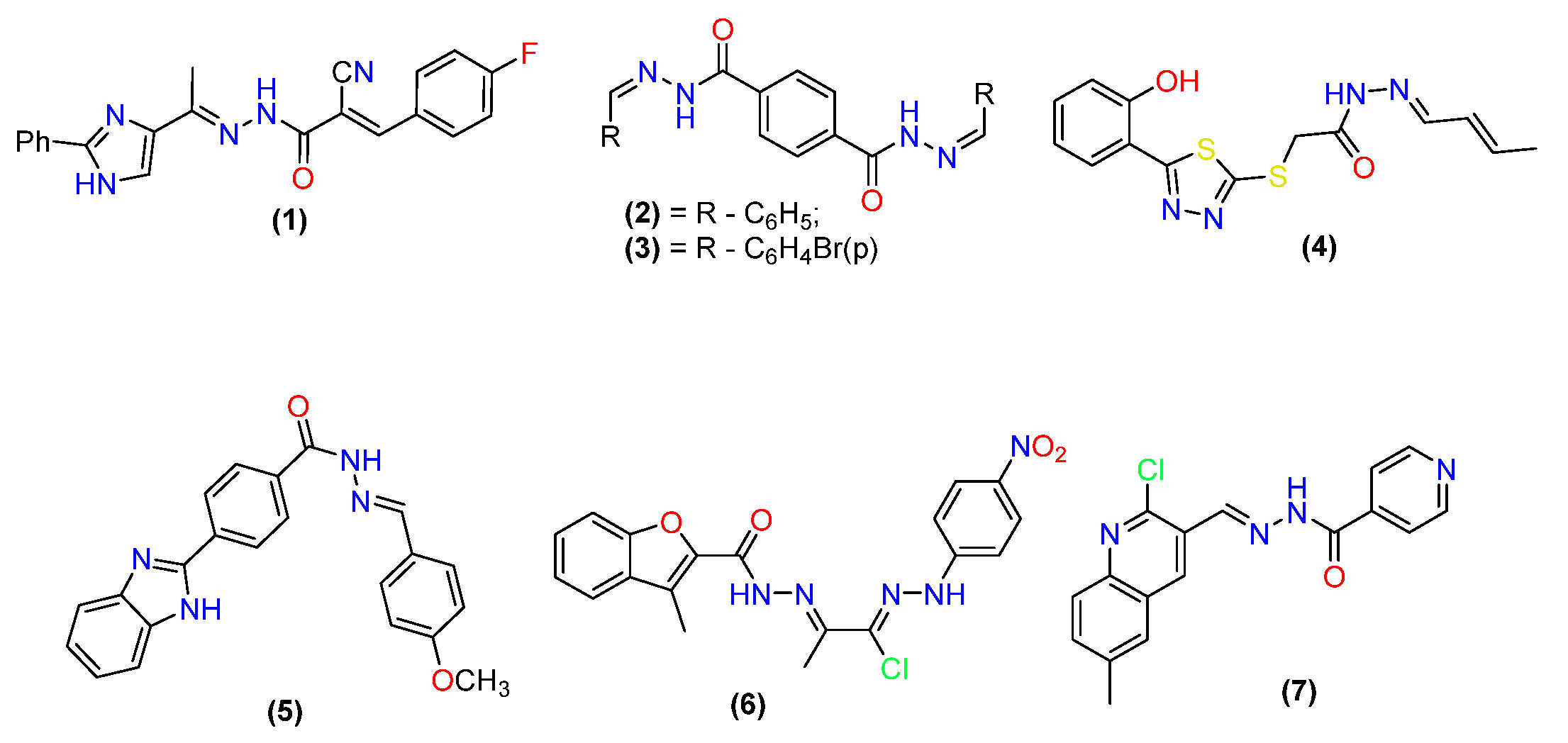
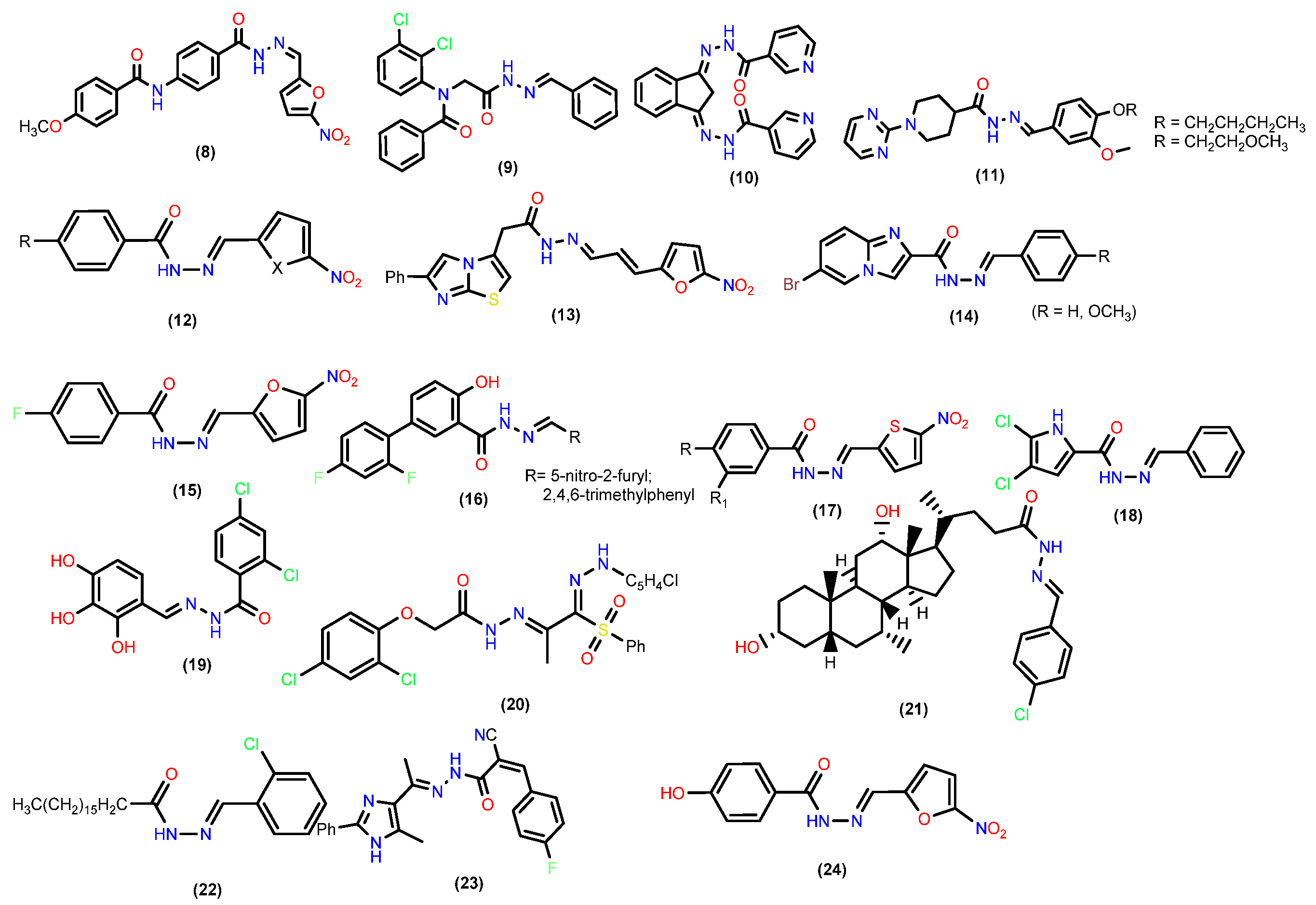
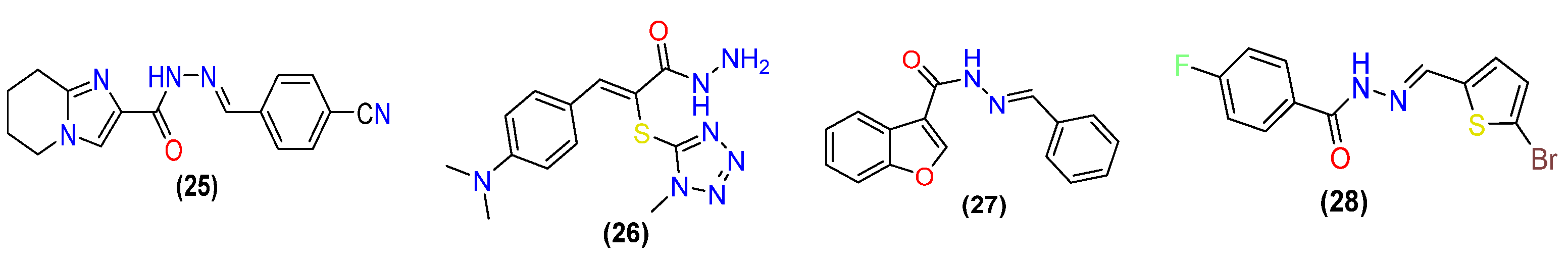
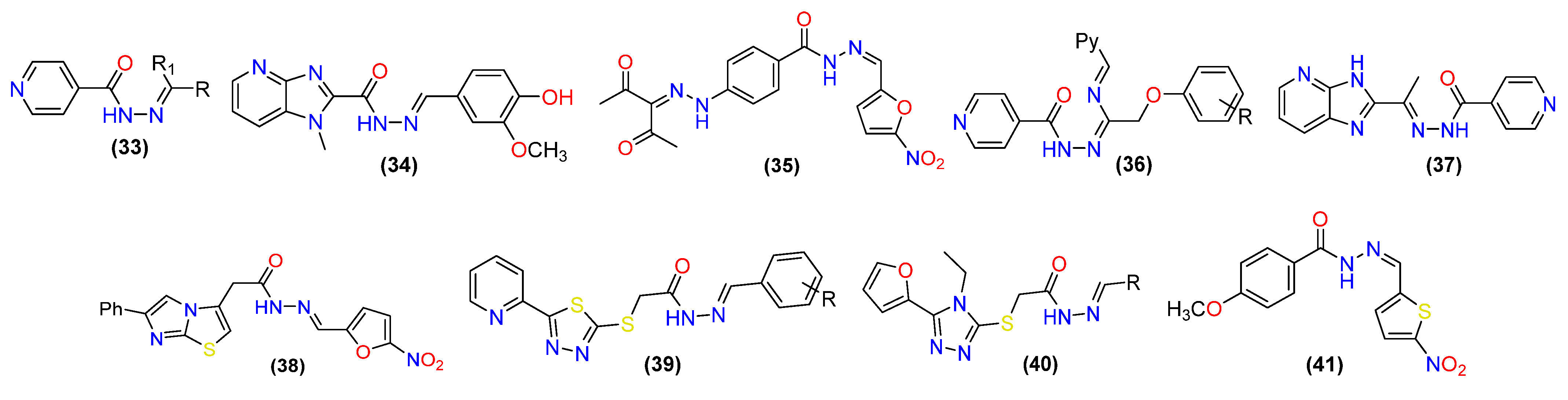
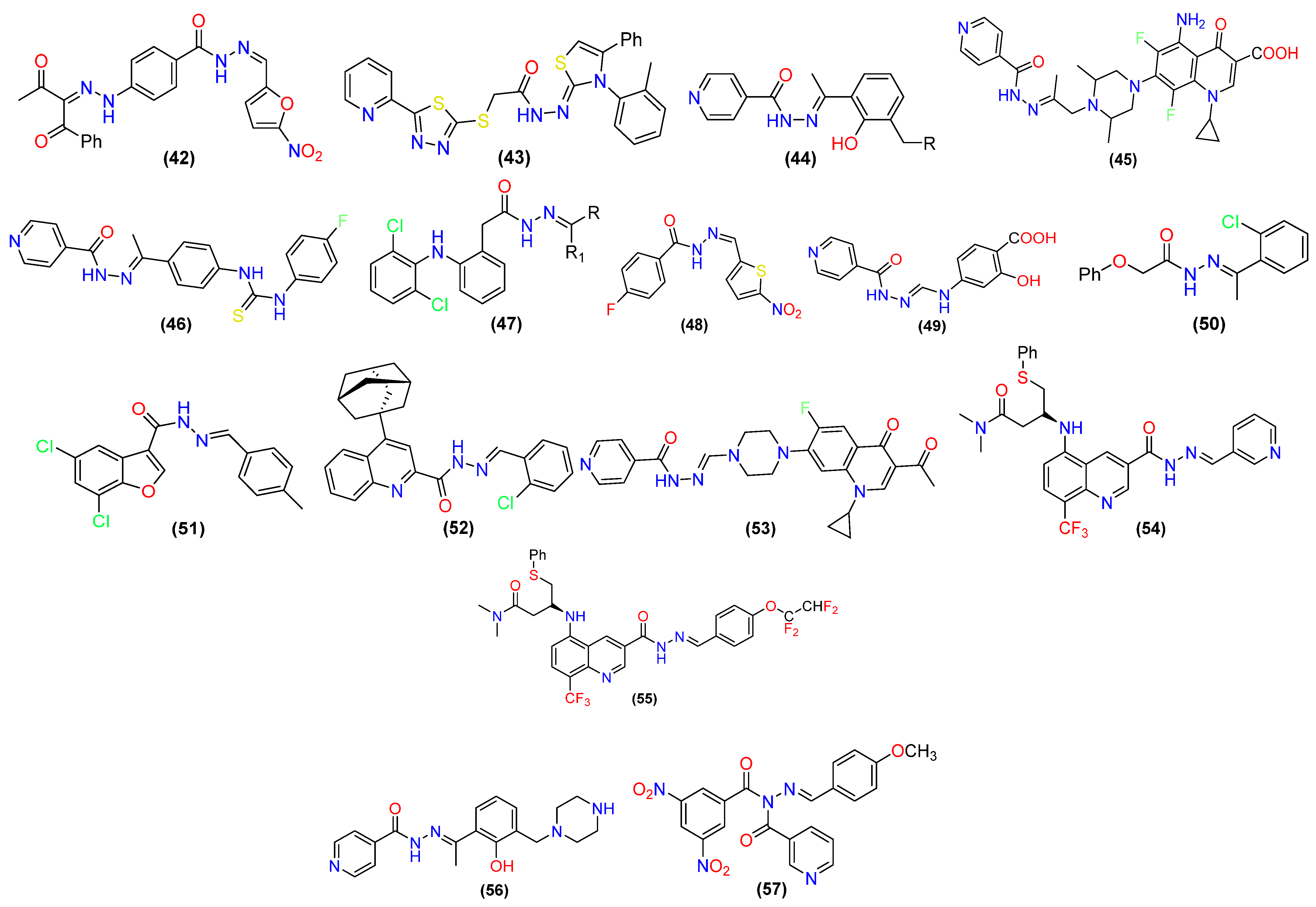
2.1. Antibacterial Activity
2.2. Anti-Fungal Activities
2.3. Antiviral Activity
2.4. Antitubercular Activity
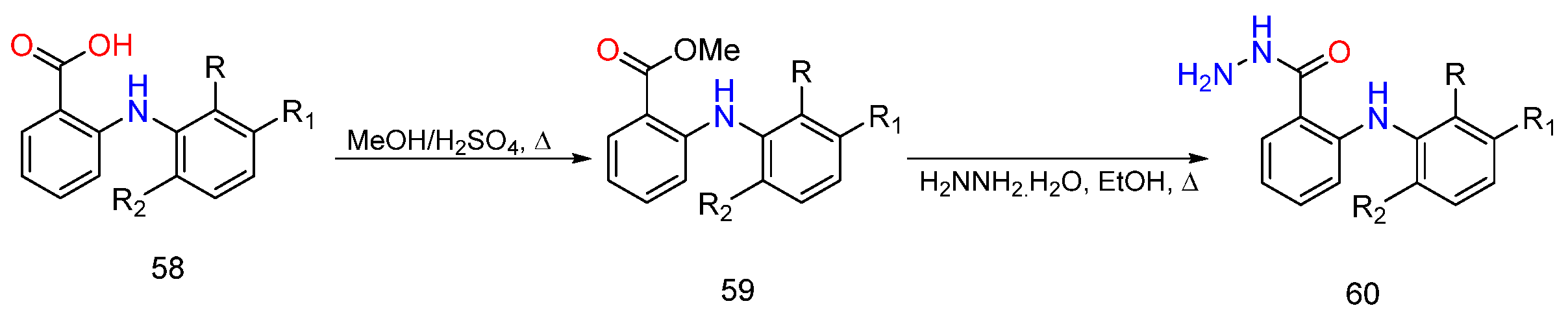
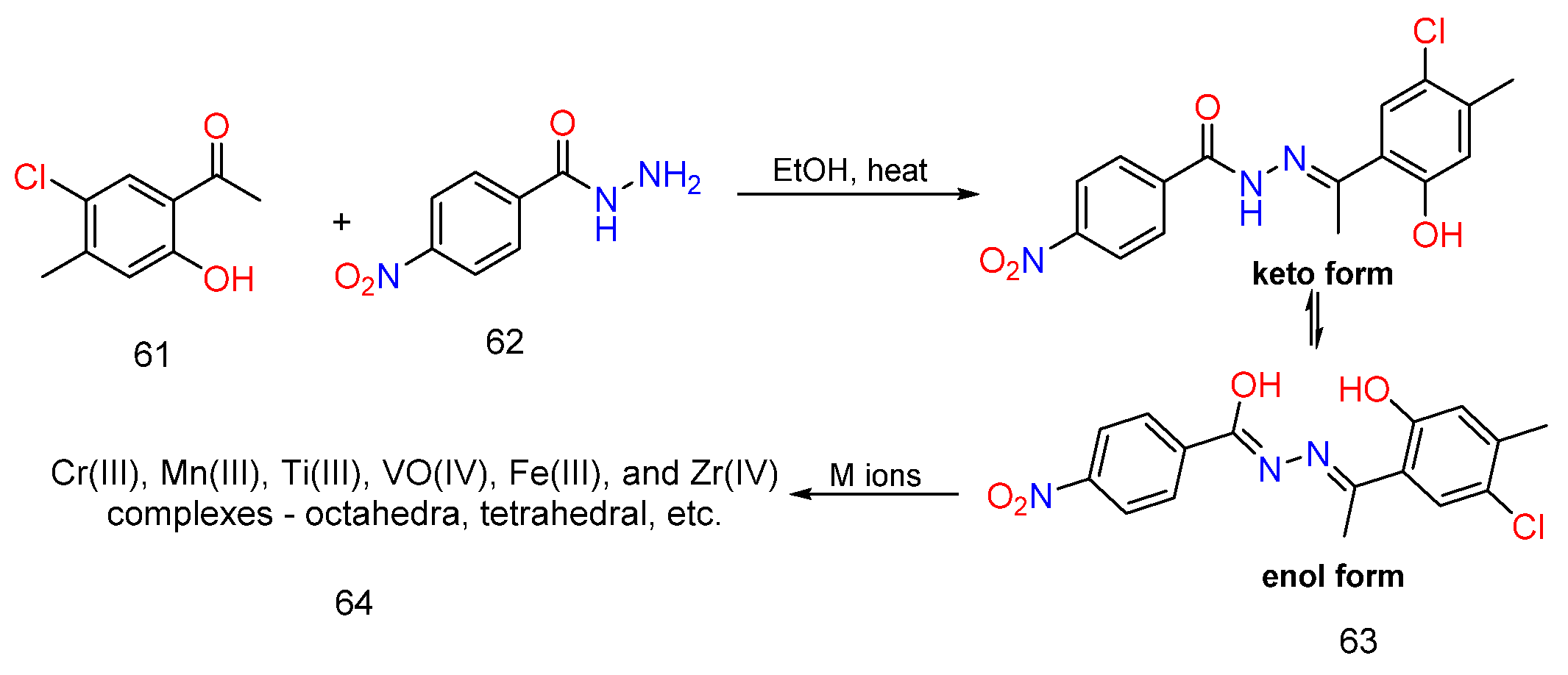
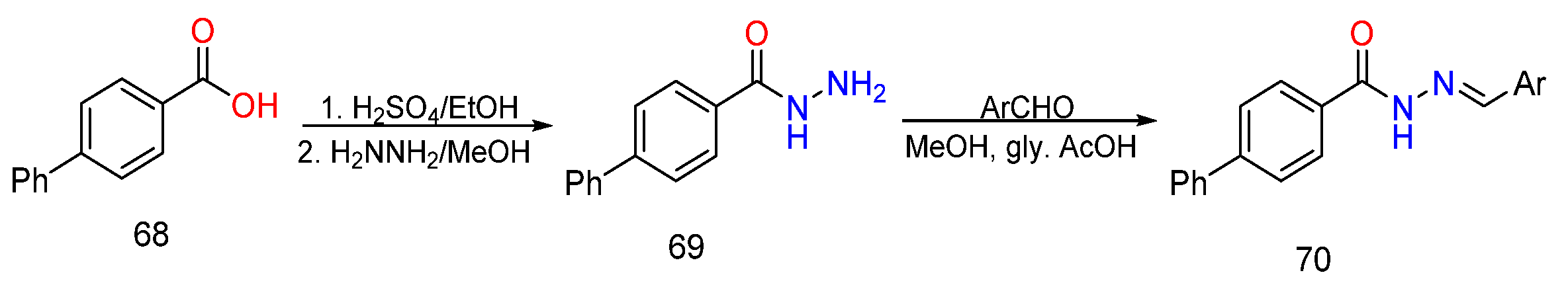
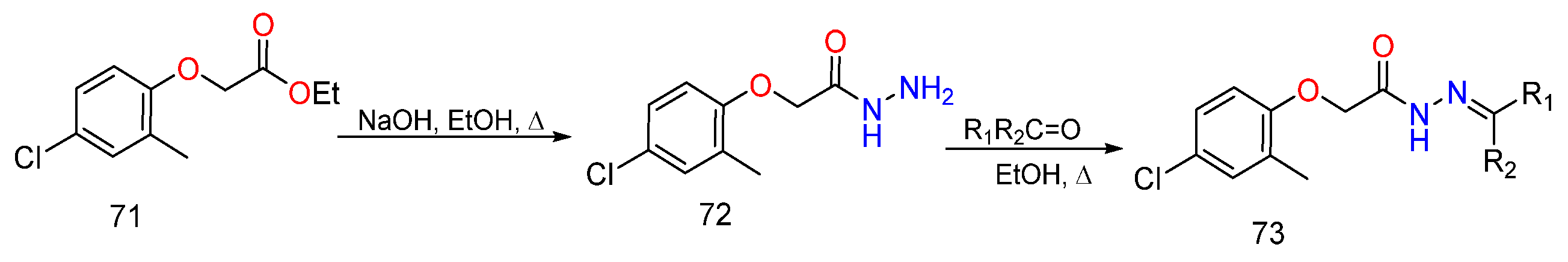
3. Different Synthetic Routes of Hydrazine, Hydrazones and Their Derivatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiyagarajan, S.; Gunanathan, C. Direct Catalytic Symmetrical, Unsymmetrical N,N-Dialkylation and Cyclization of Acylhydrazides Using Alcohols. Organ. Lett. 2020, 22, 6617–6622. [Google Scholar] [CrossRef]
- DeMarinis, R.M.; Hoover, J.R.E.; Dunn, G.L.; Actor, P.; Uri, J.V.; Weisbach, J. A new parenteral cephalosporin, SK & F 59962: Chemistry and structure activity relationships. J. Antibiot. 1975, 28, 463–470. [Google Scholar]
- Elnagdi, M.H.; Erian, A.W. New routes to polyfunctionally substituted pyridine, pyridopyridine, quinoline, and pyridazine derivatives. Arch. Pharm. 1991, 324, 853–858. [Google Scholar] [CrossRef]
- Costales, M.J.; Kleschick, W.A.; Ehr, R.J.; Weimer, M.R.U.S. N-(1-Ethyl-4-pyrazolyl)triazoloazinesulfonamide Herbicides. Patent 5,763,359, 9 June 1998. [Google Scholar]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.P.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N.; et al. Synthesis of N1-arylidene-N2-quinolyl-and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorgan. Med. Chem. Lett. 2006, 16, 5384–5388. [Google Scholar] [CrossRef] [PubMed]
- Bijev, A. New heterocyclic hydrazones in the search for antitubercular agents: Synthesis and in vitro evaluations. Lett. Drug Des. Discov. 2006, 3, 506–512. [Google Scholar] [CrossRef]
- Ragavendran, J.V.; Sriram, D.; Patel, S.K.; Reddy, I.V.; Bharathwajan, N.; Stables, J.; Yogeeswari, P. Design and synthesis of anticonvulsants from a combined phthalimide–GABA–anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 146–151. [Google Scholar] [CrossRef]
- Todeschini, A.R.; de Miranda, A.L.P.; da Silva, K.C.M.; Parrini, S.C.; Barreiro, E.J. Synthesis and evaluation of analgesic, antiinflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives. Eur. J. Med. Chem. 1998, 33, 189–199. [Google Scholar] [CrossRef]
- Ergenç, N.; Günay, N.S.; Demirdamar, R. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur. J. Med. Chem. 1998, 33, 143–148. [Google Scholar] [CrossRef]
- Deep, A.; Jain, S.; Sharma, P.C.; Verma, P.; Kumar, M.; Dora, C.P. Design and biological evaluation of biphenyl-4-carboxylic acid hydrazide-hydrazone for antimicrobial activity. Synthesis 2010, 182, 183OC. [Google Scholar]
- Masunari, A.; Tavares, L.C. A new class of nifuroxazide analogues: Synthesis of 5-nitrothiophene derivatives with antimicrobial activity against multidrug-resistant Staphylococcus aureus. Bioorgan. Med. Chem. 2007, 15, 4229–4236. [Google Scholar] [CrossRef]
- Fahmy, S.M.; Badran, A.H.; Elnagdi, M.H. Synthesis of some new azopyrazole dyes. J. Chem. Technol. Biotechnol. 1980, 30, 390–395. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Awad, G.E.; Badria, F.A. Synthesis, antimicrobial, antioxidant, anti-hemolytic and cytotoxic evaluation of new imidazole-based heterocycles. Eur. J. Med. Chem. 2011, 46, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Palekar, V.S.; Damle, A.J.; Shukla, S.R. Synthesis and antibacterial activity of some novel bis-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and bis-4-thiazolidinone derivatives from terephthalic dihydrazide. Eur. J. Med. Chem. 2009, 44, 5112–5116. [Google Scholar] [CrossRef]
- Zhong, N.J.; Wang, Y.Z.; Cheng, L.; Wang, D.; Liu, L. Recent advances in the annulation of Morita–Baylis–Hillman adducts. Organ. Biomol. Chem. 2018, 16, 5214–5227. [Google Scholar] [CrossRef] [PubMed]
- Özkay, Y.; Tunalı, Y.; Karaca, H.; Işıkdağ, İ. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur. J. Med. Chem. 2010, 45, 3293–3298. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Mekawey, A.A. Stereoselective synthesis and antimicrobial activity of benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones. Eur. J. Med. Chem. 2009, 44, 4985–4997. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Ali, M.R.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar]
- Küçükgüzel, Ş.G.; Oruç, E.E.; Rollas, S.; Şahin, F.; Özbek, A. Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1, 3, 4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002, 37, 197–206. [Google Scholar] [CrossRef]
- Asif, M. Pharmacologically potentials of hydrazonone containing compounds: A promising scaffold. Int. J. Adv. Chem. 2014, 2, 85–103. [Google Scholar] [CrossRef]
- Rollas, S.; Gulerman, N.; Erdeniz, H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Il Farmaco 2002, 57, 171–174. [Google Scholar] [CrossRef]
- Jubie, S.; Meena, S.; Ramaseshu, K.V.; Jawahar, N.; Vijayakumar, S. Synthesis and biological evaluation of some hydrazones and carbazones of indane-1,3-dione. Indian J. Chem. 2010, 49, 1261–1263. [Google Scholar]
- Govindasami, T.; Pandey, A.; Palanivelu, N.; Pandey, A. Synthesis, characterization and antibacterial activity of biologically important vanillin related hydrazone derivatives. In. J. Organ. Chem. 2011, 1, 71. [Google Scholar] [CrossRef]
- Tavares, L.C.; Chiste, J.J.; Santos, M.G.; Penna, T.C. Synthesis and biological activity of nifuroxazide and analogs. II Bollettino Chimico Farmaceutico 1999, 138, 432–436. [Google Scholar]
- Ulusoy, N.; Çapan, G.; Otük, G.; Kiraz, M. Synthesis and antimicrobial activity of new 6-phenylimidazo[2,1-b]thiazole derivatives. Bollettino Chimico Farmaceutico 2000, 139, 167–172. [Google Scholar] [PubMed]
- Turan-Zitouni, G.; Blache, Y.; Güven, K. Synthesis and antimicrobial activity of some imidazo-[1,2-a]pyridine-2-carboxylic acid arylidenehydrazide derivatives. Bollettino Chimico Farmaceutico 2001, 140, 397–400. [Google Scholar]
- Küçükgüzel, S.G.; Mazi, A.; Sahin, F.; Öztürk, S.; Stables, J. Synthesis and biological activities of diflunisal hydrazide–hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef]
- Rane, R.A.; Telvekar, V.N. Synthesis and evaluation of novel chloropyrrole molecules designed by molecular hybridization of common pharmacophores as potential antimicrobial agents. Bioorgan. Med. Chem. Lett. 2010, 20, 5681–5685. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jeong, K.W.; Shin, S.; Lee, J.U.; Kim, Y. Discovery of novel selective inhibitors of Staphylococcus aureus β-ketoacyl acyl carrier protein synthase III. Eur. J. Med. Chem. 2012, 47, 261–269. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Khidre, R.E.; Awad, G.E. Regioselective synthesis and antimicrobial activities of some novel aryloxyacetic acid derivatives. Eur. J. Med. Chem. 2012, 50, 55–62. [Google Scholar] [CrossRef]
- Ali, M.R.; Marella, A.; Alam, M.T.; Naz, R.; Akhter, M.; Shaquiquzzaman, M.; Saha, R.; Tanwar, O.; Alam, M.M.; Hooda, J. Review of biological activities of hydrazones. Indones. J. Pharm. 2012, 23, 193–202. [Google Scholar]
- Kumar, D.; Judge, V.; Narang, R.; Sangwan, S.; De Clercq, E.; Balzarini, J.; Narasimhan, B. Benzylidene/2-chlorobenzylidene hydrazides: Synthesis, antimicrobial activity, QSAR studies and antiviral evaluation. Eur. J. Med. Chem. 2010, 45, 2806–2816. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.; Siddiqi, N.J. HYDRAZONES AS PROSPECTIVE ANTI-HIV AGENT. Eur. J. Biomed. Pharm. Sci. 2016, 3, 454–458. [Google Scholar]
- Devi, T.S.; Rajitha, G. Microwave Assisted Synthesis and Evaluation of N-cinnamoyl aryl hydrazones for Cytotoxic and Antioxidant Activities. Orient. J. Chem. 2016, 32, 1703–1709. [Google Scholar] [CrossRef][Green Version]
- Altıntop, M.D.; Özdemir, A.; Turan-Zitouni, G.; Ilgın, S.; Atlı, Ö.; İşcan, G.; Kaplancıklı, Z.A. Synthesis and biological evaluation of some hydrazone derivatives as new anticandidal and anticancer agents. Eur. J. Med. Chem. 2012, 58, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Telvekar, V.N.; Belubbi, A.; Bairwa, V.K.; Satardekar, K. Novel N′-benzylidene benzofuran-3-carbohydrazide derivatives as antitubercular and antifungal agents. Bioorgan. Med. Chem. Lett. 2012, 22, 2343–2346. [Google Scholar] [CrossRef]
- Koçyiğit-Kaymakçıoğlu, B.; Oruç-Emre, E.E.; Ünsalan, S.; Tabanca, N.; Khan, S.I.; Wedge, D.E.; İşcan, G.; Demirci, F.; Rollas, S. Synthesis and biological activity of hydrazide–hydrazones and their corresponding 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles. Med. Chem. Res. 2012, 21, 3499–3508. [Google Scholar] [CrossRef]
- Hiremathad, A.; Patil, M.R.; Chethana, K.R.; Chand, K.; Santos, M.A.; Keri, R.S. Benzofuran: An emerging scaffold for antimicrobial agents. RSC Adv. 2015, 5, 96809–96828. [Google Scholar] [CrossRef]
- El-Sabbagh, O.I.; Rady, H.M. Synthesis of new acridines and hydrazones derived from cyclic β-diketone for cytotoxic and antiviral evaluation. Eur. J. Med. Chem. 2009, 44, 3680–3686. [Google Scholar] [CrossRef]
- Tian, B.; He, M.; Tang, S.; Hewlett, I.; Tan, Z.; Li, J.; Jin, Y.; Yang, M. Synthesis and antiviral activities of novel acylhydrazone derivatives targeting HIV-1 capsid protein. Bioorgan. Med. Chem. Lett. 2009, 19, 2162–2167. [Google Scholar] [CrossRef] [PubMed]
- African American Cultural Collaborative of Mercer County. Available online: https://www.taacf.com/ (accessed on 6 September 2021).
- Maccari, R.; Ottanà, R.; Vigorita, M.G. In vitro advanced antimycobacterial screening of isoniazid-related hydrazones, hydrazides and cyanoboranes: Part 14. Bioorgan. Med. Chem. Lett. 2005, 15, 2509–2513. [Google Scholar] [CrossRef]
- Shindikar, A.V.; Viswanathan, C.L. Novel fluoroquinolones: Design, synthesis, and in vivo activity in mice against Mycobacterium tuberculosis H37Rv. Bioorgan. Med. Chem. Lett. 2005, 15, 1803–1806. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Madhu, K. Synthesis and in vitro antitubercular activity of some 1-[(4-sub) phenyl]-3-(4-{1-[(pyridine-4-carbonyl) hydrazono] ethyl}phenyl) thiourea. Bioorgan. Med. Chem. Lett. 2006, 16, 876–878. [Google Scholar] [CrossRef]
- Imramovský, A.; Polanc, S.; Vinšová, J.; Kočevar, M.; Jampílek, J.; Rečková, Z.; Kaustová, J. A new modification of anti-tubercular active molecules. Bioorgan. Med. Chem. 2007, 15, 2551–2559. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V.; Pusceddu, M.C.; Schivo, M.L.; De Logu, A. Synthesis and antimycobacterial activity of some isonicotinoylhydrazones. Eur. J. Med. Chem. 1999, 34, 1071–1076. [Google Scholar] [CrossRef]
- Bukowski, L.; Janowiec, M. 1-Methyl-1H-2-imidazo [4, 5-b] pyridinecarboxylic acid and some of its derivatives with suspected antituberculotic activity. Die Pharmazie 1996, 51, 27–30. [Google Scholar] [CrossRef]
- Bukowski, L.; Janowiec, M.; Zwolska-Kwiek, Z.; Andrzejczyk, Z. Synthesis and some reactions of 2-acetylimidazo[4,5-b]pyridine. Antituberculotic activity of the obtained compounds. Die Pharmazie 1999, 54, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, İ.; Tatar, E.; Küçükgüzel, Ş.G.; Rollas, S.; De Clercq, E. Synthesis of some novel thiourea derivatives obtained from 5-[(4-aminophenoxy)methyl]-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thiones and evaluation as antiviral/anti-HIV and anti-tuberculosis agents. Eur. J. Med. Chem. 2008, 43, 381–392. [Google Scholar] [CrossRef]
- Küçükgüzel, Ş.G.; Rollas, S. Synthesis, characterization of novel coupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antimycobacterial agents. Il Farmaco 2002, 57, 583–587. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazol-2-ylthio] acetic acid arylidene-hydrazide derivatives. Il Farmaco 2001, 56, 587–592. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Banfi, L.V.E.; Scialino, G. Synthesis and antimycobacterial activity of (3,4-diaryl-3H-thiazol-2-ylidene)-hydrazide derivatives. Il Farmaco 2003, 58, 631–637. [Google Scholar] [CrossRef]
- Ulusoy, N.; Gürsoy, A.; Ötük, G. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Il Farmaco 2001, 56, 947–952. [Google Scholar] [CrossRef]
- Rando, D.G.; Sato, D.N.; Siqueira, L.; Malvezzi, A.; Leite, C.Q.; Ferreira, E.I.; Tavares, L.C. Potential tuberculostatic agents. Topliss application on benzoic acid [(5-nitro-thiophen-2-yl)-methylene]-hydrazide series. Bioorgan. Med. Chem. 2002, 10, 557–560. [Google Scholar] [CrossRef]
- Bedia, K.K.; Elçin, O.; Seda, U.; Fatma, K.; Nathaly, S.; Sevim, R.; Dimoglo, A. Synthesis and characterization of novel hydrazide–hydrazones and the study of their structure–antituberculosis activity. Eur. J. Med. Chem. 2006, 41, 1253–1261. [Google Scholar] [CrossRef]
- Saeed, A.; Bolte, M. 1-(4-Chlorophenyl)-4,4,6-trimethyl-3,4-dihydropyrimidine-2 (1H)-thione. Acta Crystallogr. Sect. E: Struct. Rep. Online 2010, 66, o440. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.S.; Agarwal, A.K.; Mahajan, N.; Pandeya, S.N.; Ananthan, S. Antibacterial and antitubercular activities of some diphenyl hydrazones and semicarbazones. Indian J. Chem. Sect. B. 2010, 49, 1384–1388. [Google Scholar]
- Rahaman, M.; Hossain, M.M. Convenient Preparation of 3-Ethoxycarbonyl Benzofurans from Salicylaldehydes and Ethyl Diazoacetate. Org. Synth. 2019, 96, 98–109. [Google Scholar] [CrossRef]
- Nayyar, A.; Monga, V.; Malde, A.; Coutinho, E.; Jain, R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of 4-(adamantan-1-yl)-2-substituted quinolines. Bioorgan. Med. Chem. 2007, 15, 626–640. [Google Scholar] [CrossRef]
- Poggi, M.; Barroso, R.; Costa-Filho, A.J.; Barbosa de Barros, H.; Pavan, F.; Queico Leite, C.; Gambino, D.; Helvecia Torre, M. New isoniazid complexes, promising agents against Mycobacterium tuberculosis. J. Mex. Chem. Soc. 2013, 57, 198–204. [Google Scholar] [CrossRef]
- Eswaran, S.; Adhikari, A.V.; Pal, N.K.; Chowdhury, I.H. Design and synthesis of some new quinoline-3-carbohydrazone derivatives as potential antimycobacterial agents. Bioorgan. Med. Chem. Lett. 2010, 20, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Madhu, K. Synthesis and in vitro and in vivo antimycobacterial activity of isonicotinoyl hydrazones. Bioorgan. Med. Chem. Lett. 2005, 15, 4502–4505. [Google Scholar] [CrossRef]
- Kumar, P.; Narasimhan, B.; Yogeeswari, P.; Sriram, D. Synthesis and antitubercular activities of substituted benzoic acid N′-(substituted benzylidene/furan-2-ylmethylene)-N-(pyridine-3-carbonyl)-hydrazides. Eur. J. Med. Chem. 2010, 45, 6085–6089. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.U. Comparative study of microwave-assisted and conventional synthesis of ibuprofen-based acyl hydrazone derivatives. Turk. J. Chem. 2013, 37, 927–935. [Google Scholar] [CrossRef]
- Narsinghani, T.; Chaturvedi, S.C. QSAR analysis of meclofenamic acid analogues as selective COX-2 inhibitors. Bioorgan. Med. Chem. Lett. 2006, 16, 461–468. [Google Scholar] [CrossRef]
- Lehmann, J.; Kraft, G. Amphiphile Verbindungen, 1. Mitt. Zur Synthese von 1-Aryl-, 1-Aroyl-und 1-Benzyl-2,3,4-5-tetrahydro-1H-1,4-benzodiazepinen. Arch. Pharmazie 1984, 317, 595–606. [Google Scholar] [CrossRef]
- Reddy, L.V.; Suman, A.; Beevi, S.S.; Mangamoori, L.N.; Mukkanti, K.; Pal, S. Design and synthesis of 1-aroyl-2-ylidene hydrazines under conventional and microwave irradiation conditions and their cytotoxic activities. J. Brazilian Chem. Soc. 2010, 21, 98–104. [Google Scholar] [CrossRef]
- Yaul, A.R.; Dhande, V.V.; Aswar, A.S. Synthesis and Characterization of Dioxotungsten (VI) and Dioxomolybdenum (VI) Complexes of Chelating Hydrazone via Their Oxoperoxo Complexes. World J. Chem. 2013, 8, 38–41. [Google Scholar]
- Gunjan, J.; Awadh, D. Synthesis and Biological Evaluation of Some Phenyl Acetic Acid Hydrazone Derivatives. Int. Res. J. Pharm. (IRJP) 2011, 2, 110–112. [Google Scholar]
- Parashar, B.; Punjabi, P.B.; Gupta, G.D.; Sharma, V.K. Synthesis of some novel N-arylhydrazone derivatives of N-phenyl anthranilic acid. Int. J. ChemTech Res. 2009, 1, 1022–1025. [Google Scholar]
- Jamil, W.; Perveen, S.; Shah, S.A.A.; Taha, M.; Ismail, N.H.; Perveen, S.; Ambreen, N.; Khan, K.M.; Choudhary, M.I. Phenoxyacetohydrazide Schiff bases: β-Glucuronidase inhibitors. Molecules 2014, 19, 8788–8802. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mali, S.N.; Thorat, B.R.; Gupta, D.R.; Pandey, A. Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Eng. Proc. 2021, 11, 21. https://doi.org/10.3390/ASEC2021-11157
Mali SN, Thorat BR, Gupta DR, Pandey A. Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Engineering Proceedings. 2021; 11(1):21. https://doi.org/10.3390/ASEC2021-11157
Chicago/Turabian StyleMali, Suraj N., Bapu R. Thorat, Deepa Rani Gupta, and Anima Pandey. 2021. "Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity" Engineering Proceedings 11, no. 1: 21. https://doi.org/10.3390/ASEC2021-11157
APA StyleMali, S. N., Thorat, B. R., Gupta, D. R., & Pandey, A. (2021). Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Engineering Proceedings, 11(1), 21. https://doi.org/10.3390/ASEC2021-11157




