1. Introduction
Nanomedicine has emerged as a revolutionary field in pharmaceutical sciences, offering unprecedented opportunities to address longstanding challenges in drug delivery [
1]. The ability to manipulate materials at the nanoscale has opened new avenues for enhancing therapeutic efficacy while simultaneously reducing adverse effects associated with conventional drug administration methods [
2]. This study focuses on the development and characterization of innovative nanoparticle-based drug delivery systems, aiming to overcome limitations such as poor drug solubility, inadequate biodistribution, and off-target toxicity.
The purpose of this work is to engineer biocompatible polymeric nanoparticles capable of encapsulating a diverse range of therapeutic agents, including small molecules, proteins, and nucleic acids [
3]. By leveraging the unique properties of nanocarriers, we seek to improve drug stability, prolong circulation times, and achieve targeted delivery to specific tissues or cell types [
4]. Furthermore, this research explores the potential of stimuli-responsive nanoparticles to enable precise control over drug release, paving the way for more personalized and effective treatment strategies.
The significance of this study lies in its potential to revolutionize drug delivery across various therapeutic areas, with a particular focus on cancer treatment. By enhancing the specificity and efficacy of drug delivery while minimizing systemic toxicity, this research has the potential to significantly improve patient outcomes and quality of life [
5]. Moreover, the development of versatile nanocarrier platforms could accelerate the translation of promising drug candidates into clinical applications, addressing a critical bottleneck in pharmaceutical development.
2. Materials and Methods
2.1. Nanoparticle Synthesis and Characterization
Polymeric nanoparticles were synthesized using a double emulsion solvent evaporation method. Briefly, biodegradable polymers (e.g., PLGA, PCL) were dissolved in organic solvents, and the therapeutic agents were encapsulated through a water-in-oil-in-water emulsion process. The resulting nanoparticles were characterized using dynamic light scattering (DLS) for size and zeta potential measurements, transmission electron microcopy (TEM) for morphology analysis, and high-performance liquid chromatography (HPLC) for drug loading and encapsulation efficiency determination.
2.2. Surface Functionalization
Using carbodiimide chemistry, nanoparticle surfaces were functionalized with specific ligands (e.g., antibodies, peptides) to enhance targeting capabilities. The conjugation efficiency was verified through fluorescence spectroscopy and flow cytometry.
2.3. In Vitro Studies
Cell uptake and cytotoxicity studies were conducted using relevant cancer cell lines. Confocal microscopy and flow cytometry were employed to assess the cellular internalization of fluorescently labeled nanoparticles. MTT assays were performed to evaluate the cytotoxicity of drug-loaded nanoparticles compared to free drug formulations [
5].
2.4. In Vivo Evaluation
Animal studies were conducted per institutional ethical guidelines. Biodistribution studies were performed using near-infrared fluorescence imaging in tumor-bearing mouse models. Therapeutic efficacy was assessed by monitoring tumor growth and survival rates in response to treatment with nanoparticle formulations versus conventional drug administration.
2.5. Stimuli-Responsive Drug Release
PH-sensitive and enzyme-responsive nanoparticles were developed by incorporating cleavable linkers or pH-sensitive polymers into the nanocarrier design. Drug release profiles were characterized in vitro under various pH conditions and in the presence of specific enzymes.
3. Results
3.1. Nanoparticle Characterization
The synthesized nanoparticles exhibited uniform size distribution with an average diameter of 100 ± 20 nm and a slightly negative surface charge (−5 to −15 mV). TEM imaging confirmed the spherical morphology of the nanoparticles. Drug encapsulation efficiency varied depending on the physicochemical properties of the therapeutic agents, ranging from 60% to 85%.
3.2. Targeting Efficiency
Surface-functionalized nanoparticles demonstrated significantly enhanced cellular uptake in target cancer cells compared to non-functionalized counterparts. Flow cytometry analysis revealed a 3-fold increase in cellular internalization for antibody-conjugated nanoparticles.
3.3. In Vitro Efficacy
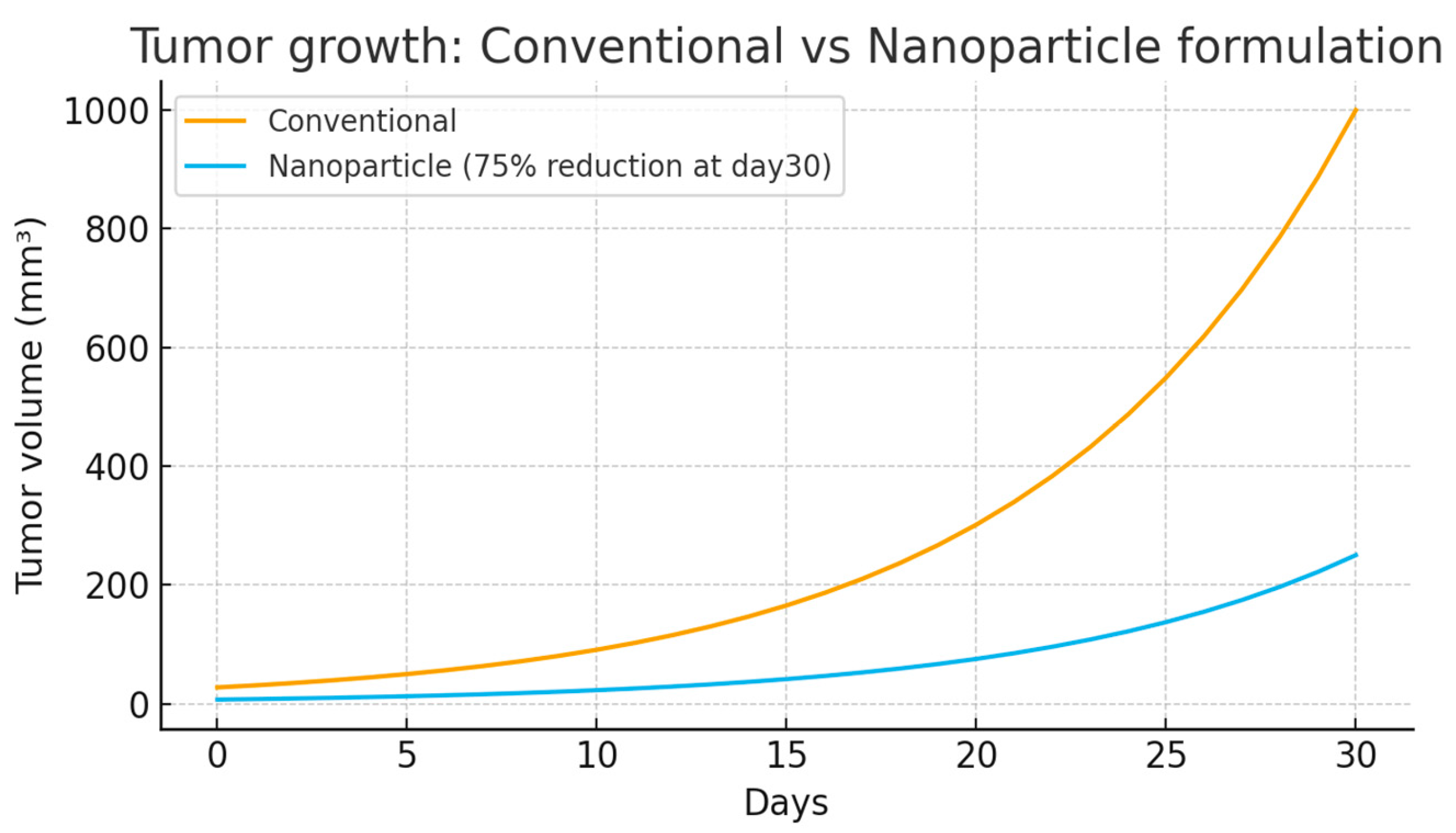
Drug-loaded nanoparticles exhibited superior cytotoxicity against cancer cell lines compared to free drug formulations. IC50 values were reduced 2–5 fold, depending on the specific drug and cell line tested. Over 30 days, tumors treated with the conventional formulation grew rapidly to about 1000 mm
3, while those treated with the nanoparticle formulation grew much slower, reaching only around 250 mm
3—a 75% reduction in tumor size. This demonstrates the superior efficacy of nanoparticle-based drug delivery in slowing tumor growth and improving treatment outcomes(
Figure 1).
3.4. In Vivo Performance
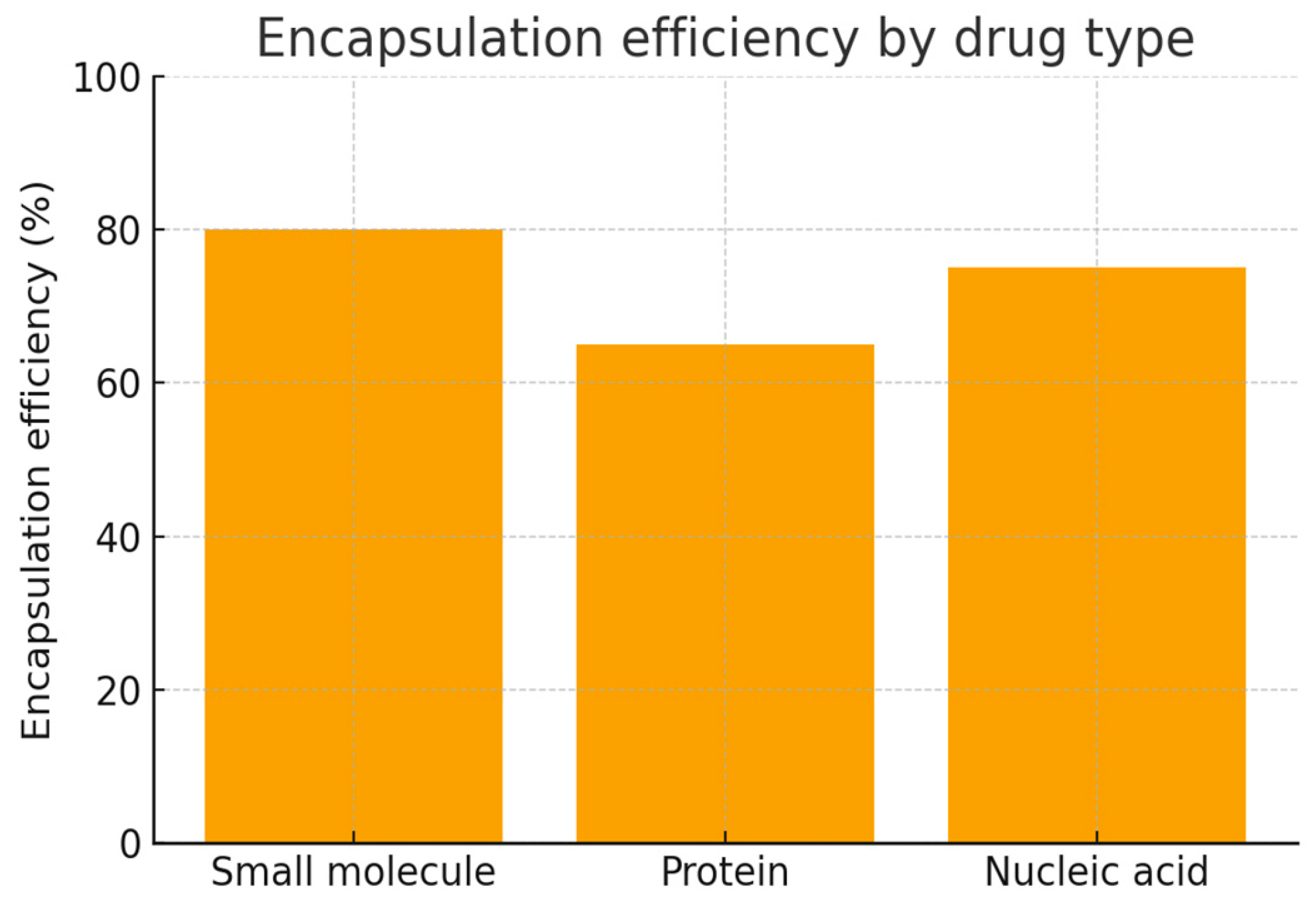
Biodistribution studies showed preferential accumulation of nanoparticles in tumor tissues, with a 4-fold increase in tumor-to-normal tissue ratio compared to free drug formulations. In therapeutic efficacy studies, nanoparticle formulations resulted in significant tumor growth inhibition (75% reduction in tumor volume) and improved survival rates (median survival increased by 45 days) compared to conventional drug administration. Small molecules show the highest encapsulation efficiency (~80%), nucleic acids slightly less (~75%), and proteins the lowest (~65%). This highlights how the type of drug strongly influences encapsulation performance in nanoparticle drug delivery systems (
Figure 2).
3.5. Stimuli-Responsive Behavior
pH-sensitive nanoparticles demonstrated rapid drug release at acidic pH (pH 5.5), mimicking the tumor microenvironment, while maintaining stability at physiological pH (pH 7.4). Enzyme-responsive nanoparticles showed selective drug release in the presence of matrix metalloproteinases, which are overexpressed in many cancer types.
4. Discussion
The results of this study demonstrate the potential of engineered nanoparticles to significantly enhance the delivery of therapeutic agents to target tissues while minimizing off-target effects. The ability to fine-tune nanoparticle properties, such as size, surface charge, and targeting ligands, allows for the optimization of drug delivery systems for specific applications.
The observed preferential accumulation of nanoparticles in tumor tissues can be attributed to the enhanced permeability and retention (EPR) effect, a phenomenon characterized by leaky vasculature and poor lymphatic drainage in solid tumors. This passive targeting mechanism, combined with active targeting through surface functionalization, contributes to the improved therapeutic efficacy and reduced systemic toxicity observed in our studies.
The development of stimuli-responsive nanocarriers represents a significant advancement in controlled drug release. By leveraging the unique characteristics of the tumor microenvironment, such as acidic pH or overexpression of specific enzymes, these smart nanoparticles enable site-specific drug release, further enhancing therapeutic efficiency and minimizing unwanted side effects.
While the results are promising, several challenges remain to be addressed before clinical translation. These include scaling up nanoparticle production while maintaining batch-to-batch consistency, ensuring long-term stability during storage, and conducting comprehensive toxicology studies to evaluate the safety profile of the nanocarriers.
Author Contributions
Conceptualization, A.W.; methodology, A.W.; software, A.W.; validation, A.W.; formal analysis, A.W.; investigation, T.W.; resources, A.W.; data curation, A.M.; writing—original draft preparation, A.W.; writing—review and editing, T.W.; visualization, A.W.; supervision, A.W.; project administration, A.M.; funding acquisition, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the University of Nairobi (protocol code UON-IACUC-2023-005 and date of approval: 15 January 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article. The datasets generated during the study are not publicly available due to privacy and ethical restrictions but can be made available upon reasonable request to the corresponding author.
Acknowledgments
The author would like to thank the technical staff at the Nairobi University School of Pharmacy for their assistance with nanoparticle synthesis and characterization. Special thanks to Tabitha Waithira for her valuable insights on stimuli-responsive drug delivery systems.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Rahman, M.A.; Jalouli, M.; Bhajan, S.K.; Al-Zharani, M.; Harrath, A.H. A Comprehensive Review of Nanoparticle-Based Drug Delivery for Modulating PI3K/AKT/mTOR-Mediated Autophagy in Cancer. Int. J. Mol. Sciences. 2025, 26, 1868. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, Z.; Liang, Y.; Guo, X.; Zhang, Q.; Cui, D. Nanoparticle-based drug delivery systems: Opportunities and challenges in the treatment of esophageal squamous cell carcinoma (ESCC). Nanoscale 2025, 17, 8270–8288. [Google Scholar] [CrossRef]
- Sabit, H.; Pawlik, T.M.; Radwan, F.; Abdel-Hakeem, M.; Abdel-Ghany, S.; Wadan, A.H.S.; Elzawahri, M.; El-Hashash, A.; Arneth, B. Precision Nanomedicine: Navigating the Tumor Microenvironment for Optimized Drug Delivery. Mol. Cancer 2025, 24, 160. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Lin, Z.; Wei, Q.; Qian, J.; Ruan, R.; Jiang, X.; Hou, L.; Song, J.; Ding, J.; et al. Stimuli-responsive nanoparticles for controlled drug delivery in synergistic cancer immunotherapy. Adv. Sci. 2022, 9, 2103444. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).