1. Introduction
Skin plays a pivotal role in the development of wearable optical sensors. The aftermath of COVID-19 brought about realization of the inaccuracies of pulse oximeters in measuring the peripheral oxygen saturation levels
in darker-skinned patients [
1]. Sdjoding et al. [
2] demonstrated that 88 out of 749 (11.7%) dark-toned patients were misdiagnosed as having normal
levels ranging between 92% to 96%, while they were actually experiencing occult hypoxemia. The same tests showed that 99 out of 2778 (3.6%) of light-toned patients at the University of Michigan Hospital suffered from a similar diagnostic error.
The main chromophores responsible for the behaviour of light on the skin are water, melanin, and haemoglobin [
3,
4,
5,
6]. Melanin, which is responsible for the pigmentation of the skin, or the skin tone, is recognised as the main absorber of light [
7]. The interaction of oxygenated and deoxygenated haemoglobin with light is used in the estimation of
.
Green, red, and infrared (IR) light-emitting diodes (LEDs) are commonly found in commercial optical wearable devices. In [
8], an in-depth discussion is given on light sources, including their skin penetration depths. They used a combination of red and green LEDs to improve the detection of their PPG sensor. Another study [
9] used red and IR LEDs with a concave housing structure and a phototransistor as the detector for finger monitoring. The calculation of
in these articles was based on the ratio (
R) of the red and green LEDs using their AC and DC values, referring to the pulsatile and non-pulsatile light absorption characteristics of the arteries and tissues present, and their molar extinction coefficient (
) [
8,
10]. Kim et al. [
11] suggested that although theoretically, skin colour does not affect the accuracy of the
measurement, the crosstalk which occurs at the surface of the skin influences the AC/DC ratio, leading to errors in the calculation of the
value. From the Beer–Lambert Law [
8,
10],
is affected by the ratio of absorptivity of the light sources,
R.
In 2020, Ellis Monk introduced a 10-shade scale as an improvement to the Fitzpatrick Scale, which has been widely used as the gold standard to classify different skin tones over the years [
12]. The previous 6-shade scale was suggested to have overlooked some skin tones, leading to occasionally incorrect identifications when used in artificial algorithms for facial recognition. The colour of an object, including skin, can be expressed numerically through colour science. The Commission Internationale de l’Éclairage (CIE) XYZ color space was adopted in this study to classify the volunteers using an objective method as opposed to the popular subjective method of visual assessment and categorisation of skin tone to match the Fitzpatrick scale.
This research observed the reSpOnsivity of 12 different skin tones to a white light source within the visible spectral range of 300–700 nm. It was noted that darker-skin tones responded differently to wavelengths within the indigo-blue band and the yellow-orange band of the spectrum as compared to lighter-skin tones.
2. Materials and Methods
2.1. Participant Selection
Twelve individuals with an even distribution of light to dark tones, comprising six females and six males within an age range of 20 to 50, were randomly selected from the University of Pretoria. Each of the participants was notified of the risks involved with the experiments as guided by the Ethics Committee of the University of Pretoria, and indemnity forms were signed for consent.
2.2. Instruments
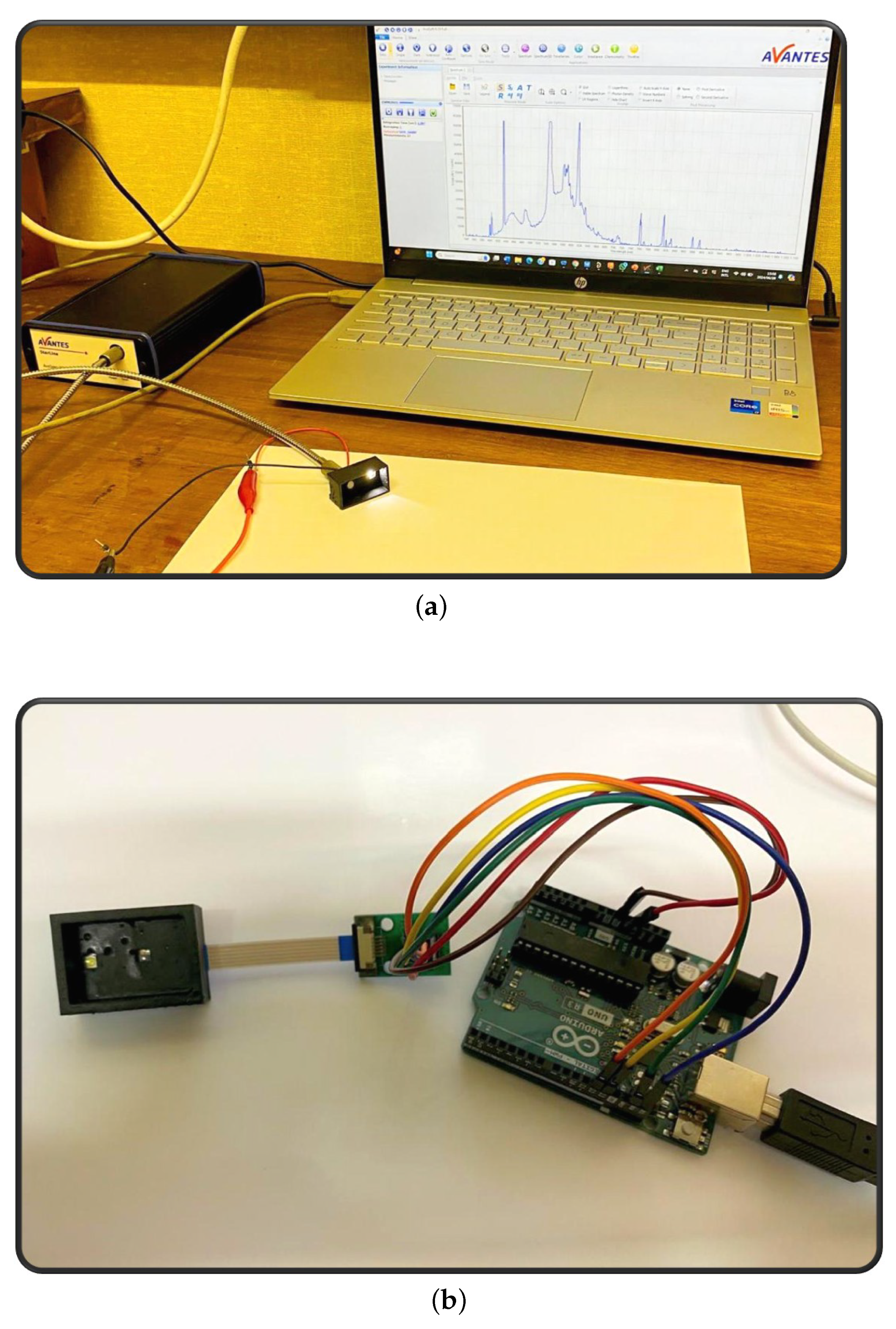
A white light-emitting diode (LED) with a frequency range of 300 to 700 nm was used as the light source for all the experiments. The initial series of reflectance measurements was conducted utilising a Starline AvaSpec 2048 Spectrometer, equipped with a CC-VIS/NIR cosine corrector connected to the FC-UVIR600-2-BX optical cable; all components were manufactured by Avantes, Apeldoorn, The Netherlands. This setup was then changed to an 11-Channel Adrafruit AS7341 spectral colour sensor circuit, which was printed on a flexible Kapton substrate. An Arduino Uno R3 board was used as an interface between the sensor and the Python, version 3.12 script for processing the data.
The reflected white light from the skin of the volunteers was measured using the two experimental setups, as seen in
Figure 1. The optical sensors were placed on the inner wrist of the participants, which appeared to be less exposed to the tanning effects of the sun. The wrists were cleaned with alcohol wipes on the area of interest to ensure that the surface was free from debris, as well as body lotion or creams, which could influence the behaviour of the illuminated light.
All measurements were carried out in a dark laboratory to minimize ambient light interference. The measurement setups were housed in a 3D-printed case, which was developed using the software Fusion 360 and printed on a stereolithographical Formlabs 3/3A printer using black resin. The casing ensured that for all readings taken, the photodetector was 1 cm from the surface of the skin, as well as 1 cm apart from the light source. The room temperature measured between 19.5 and 20.5 °C.
3. Results
3.1. Classification of Skin Tones
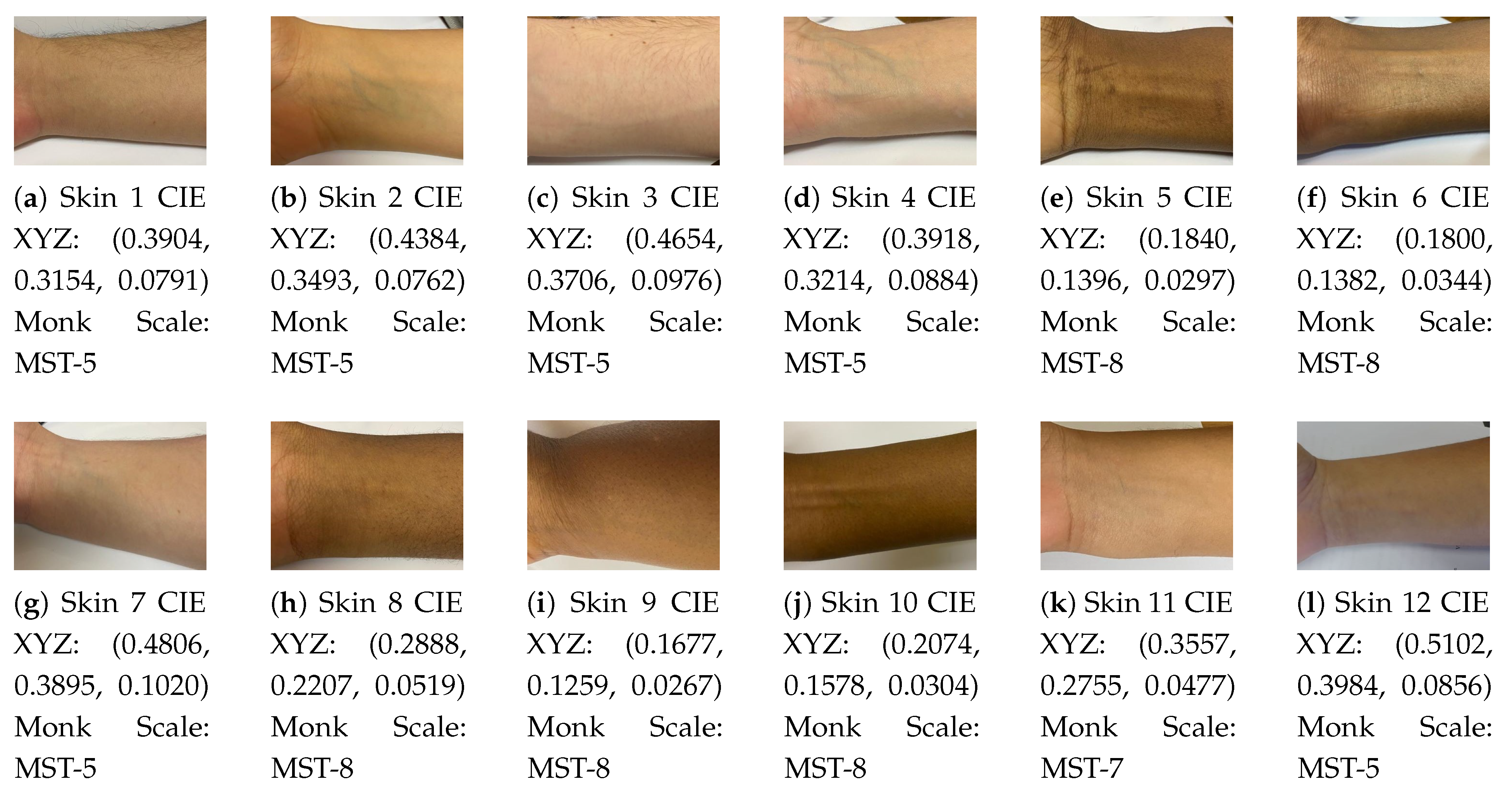
Figure 2 illustrates the 12 skin tones which were measured as well as the CIE XYZ colour output. It categorizes the skin tones into the corresponding Monk scale equivalents.
3.2. Spectrum Analysis of Different Skin Tones
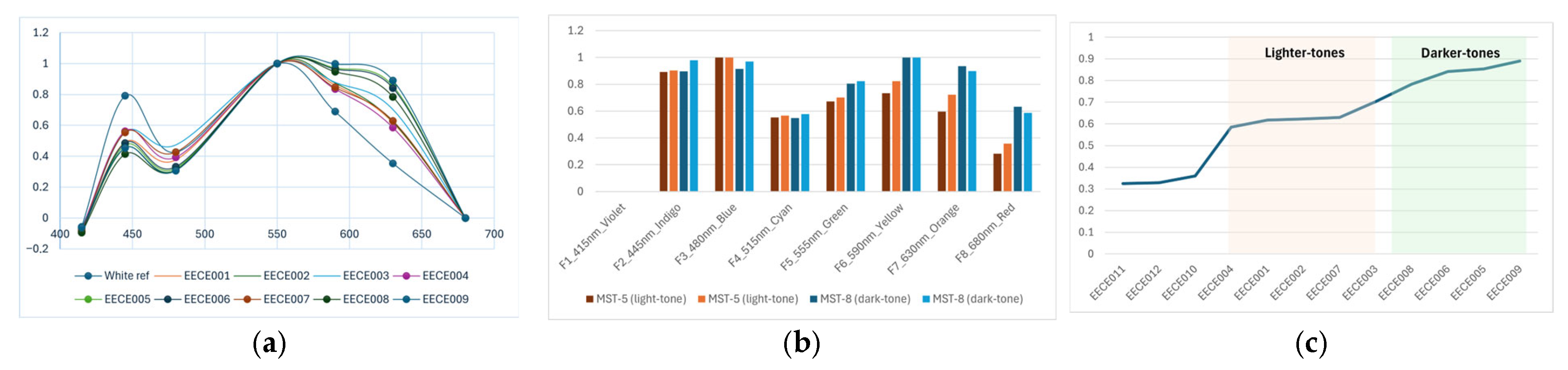
The normalised results of the reflectance measurements using both the commercial spectrometer and the AS7341 sensor manufactured by Avantes, Apeldoorn, The Netherlands showed that lighter skin tones reflected light in the shorter wavelengths of 450 nm and absorbed more light in the longer wavelengths between 575 and 650 nm. The opposite was noted in the darker skin tones. Although these wavelengths are subject to haemoglobin, the resulting pattern in
Figure 3 identifies a distinct grouping according to skin tone, indicating that there is a presence of influencing factors at those wavelengths other than just haemoglobin.
4. Discussion
The CIE XYZ values measured provided a distinction between the skin tones of the participants, enabling them to be grouped according to light tones (CIE XYZ = 0.4, 0.3, 0.08) and darker tones (CIE XYZ = 0.2, 0.1, 0.03). The accuracy of the colour was influenced by the red shade which appeared on the surface of the skin when the white LED was illuminated 1 cm from the surface. This was more evident in lighter skin tones. Further data manipulation is required to remove this effect to obtain melanin information aligned to the skin colour scale.
Although the Monk Scale is a welcome upgrade to the Fitzpatrick scale, it still faces limitations as it provides discrete tones which do not apply in real-life situations. An electronic definition of skin tone through wavelength fingerprinting could provide a broader range of skin colour, increasing the accuracy of recognition patterns.
The study showed that darker skin tones reflected more light between wavelengths of 550 and 650 nm, producing a flatter gradient as compared to lighter tones, which reflected more at the blue wavelength of 450 nm. The shorter wavelength absorption of darker tones could be explained by the presence of an increased concentration of melanin in the skin, resulting in more photons being absorbed. However, a larger sample size, inclusive of a wider variety of skin tones, would provide a more detailed analysis.
Since is calculated using the ratio, R, of absorptivity of red light over the absorptivity of green light, if the red light DC, factoring in the effect of skin pigmentation, decreases, R decreases, resulting in an overall increase in the final . Apart from the crosstalk mentioned in the introduction, this could be the cause for the exceptionally higher values in darker-toned individuals. The change in DC is influenced by skin pigmentation.
The manufacturing readiness level of this research is at level 3, whereby laboratory experiments were conducted to provide proof of concept. Future work will involve using these findings in developing a personalised sensor which would improve the accuracy of pulse oximeter diagnosis.
Author Contributions
Conceptualization, methodology, software, validation, forrmal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, N.N.N.; supervision, project administration, and funding acquisition, T.-H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the South African Department of Science and Innovation Nano and Micro Manufacturing Facility grant.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the University of Pretoria (protocol code EBIT/247/2022 and 4 July 2023).
Informed Consent Statement
Informed consent for participation was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Plaisime, M.V. Invited Commentary: Undiagnosed and undertreated—The suffocating consequences of the use of racially biased medical devices during the COVID-19 pandemic. Am. J. Epidemiol. 2023, 195, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Sjoding, M.W.; Dickson, R.P.; Iwashyna, T.J.; Gay, S.E.; Thomas, S.; Valley, T.S. Racial Bias in Pulse Oximetry Measurement. NEJM 2020, 383, 2477–2478. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E. Understanding the color of human skin. IS&T/SPIE Electron. Imaging 2001, 4299, 243–251. [Google Scholar] [CrossRef]
- Kollias, N.; Baqer, A. An experimental study of the changes in pigmentation in human skin in vivo with visible and near-infrared light. Photochem. Photobiol. 1984, 39, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Zonios, G.; Bykowski, J.; Kollias, N. Skin Melanin, Hemoglobin, and Light Scattering Properties can be Quantitatively Assessed In Vivo Using Diffuse Reflectance Spectroscopy. JID 2001, 117, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Piazena, H.; Meffert, H.; Uebelhack, R. Spectral Remittance and Transmittance of Visible and Infrared-A Radiation in Human Skin—Comparison Between in vivo Measurements and Model Calculations. Photochem. Photobiol. 2017, 93, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Koerber, D.; Khan, S.; Shamsheri, T.; Kirubarajan, A.; Mehta, S. Accuracy of Heart Rate Measurement with Wrist-Worn Wearable Devices in Various Skin Tones: A Systematic Review. J. Racial Ethn. Health Disparities 2022, 10, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- Banik, P.P.; Hossain, S.; Kwon, T.H.; Kim, H.; Kim, K.D. Development of a Wearable Reflection-Type Pulse Oximeter System to Acquire Clean PPG Signals and Measure Pulse Rate and SpO2 with and without Finger Motion. Electronics 2020, 9, 1905. [Google Scholar] [CrossRef]
- Pang, G.; Ma, C. A Neo Reflective Wrist Pulse Oximeter. IEEE Access 2014, 2, 1562–1567. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Wei, Z. Continuous blood oxygen estimation using PPG based on VMD. Electronics 2020, 9, 1905. [Google Scholar] [CrossRef]
- Kim, K.B.; Baek, H.J. Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics 2023, 12, 2923. [Google Scholar] [CrossRef]
- Teaching Algorithms About Skin Tones. Available online: https://news.harvard.edu/gazette/story/2022/07/teaching-algorithms-about-colors-of-people/ (accessed on 17 May 2023).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).