Development of a Nano- and Microfiber Mesh-Based Biosensor for the Rapid Quantification of Human C-Reactive Protein (CRP) †
Abstract
1. Introduction
2. Materials and Methods
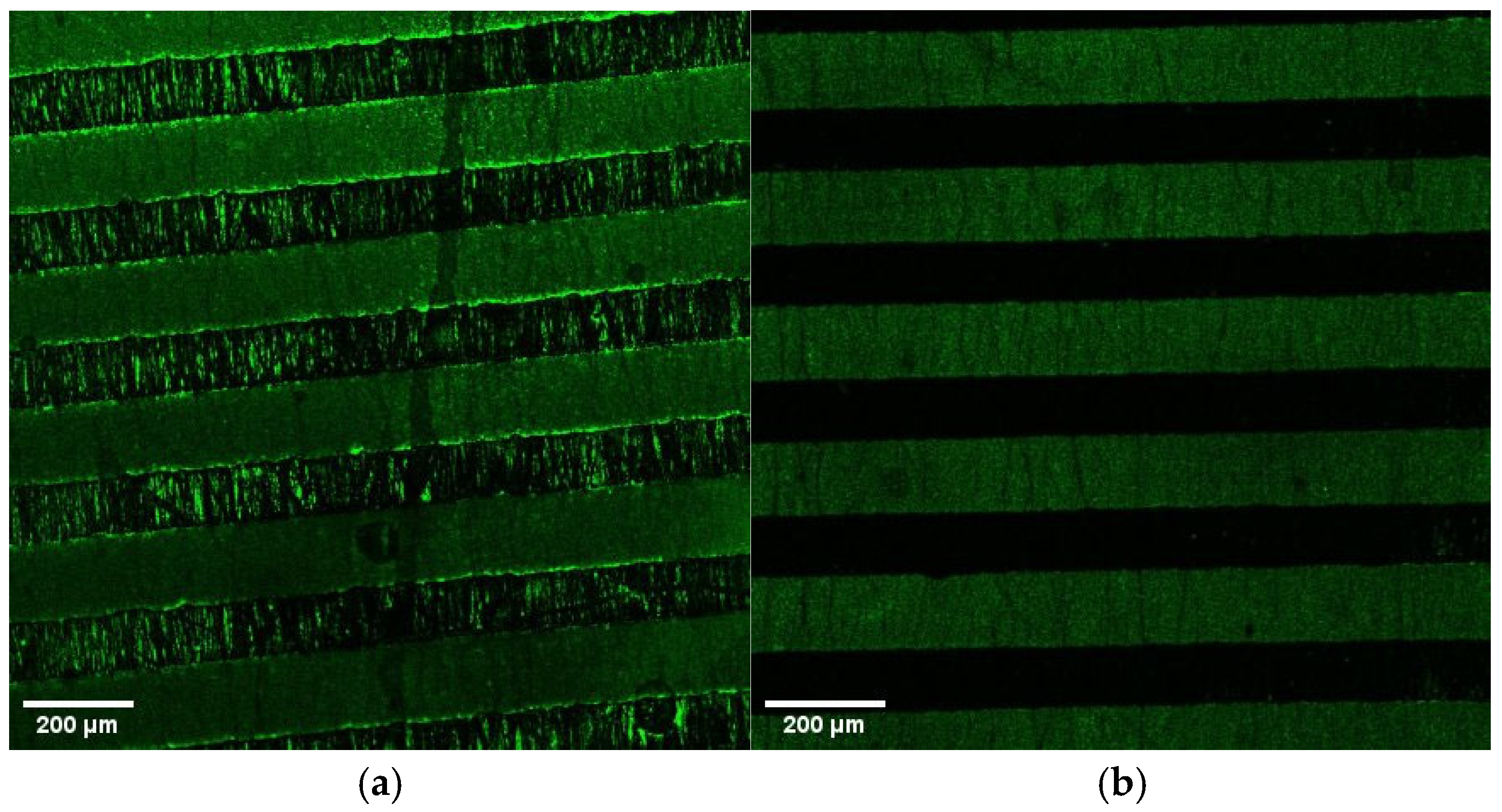
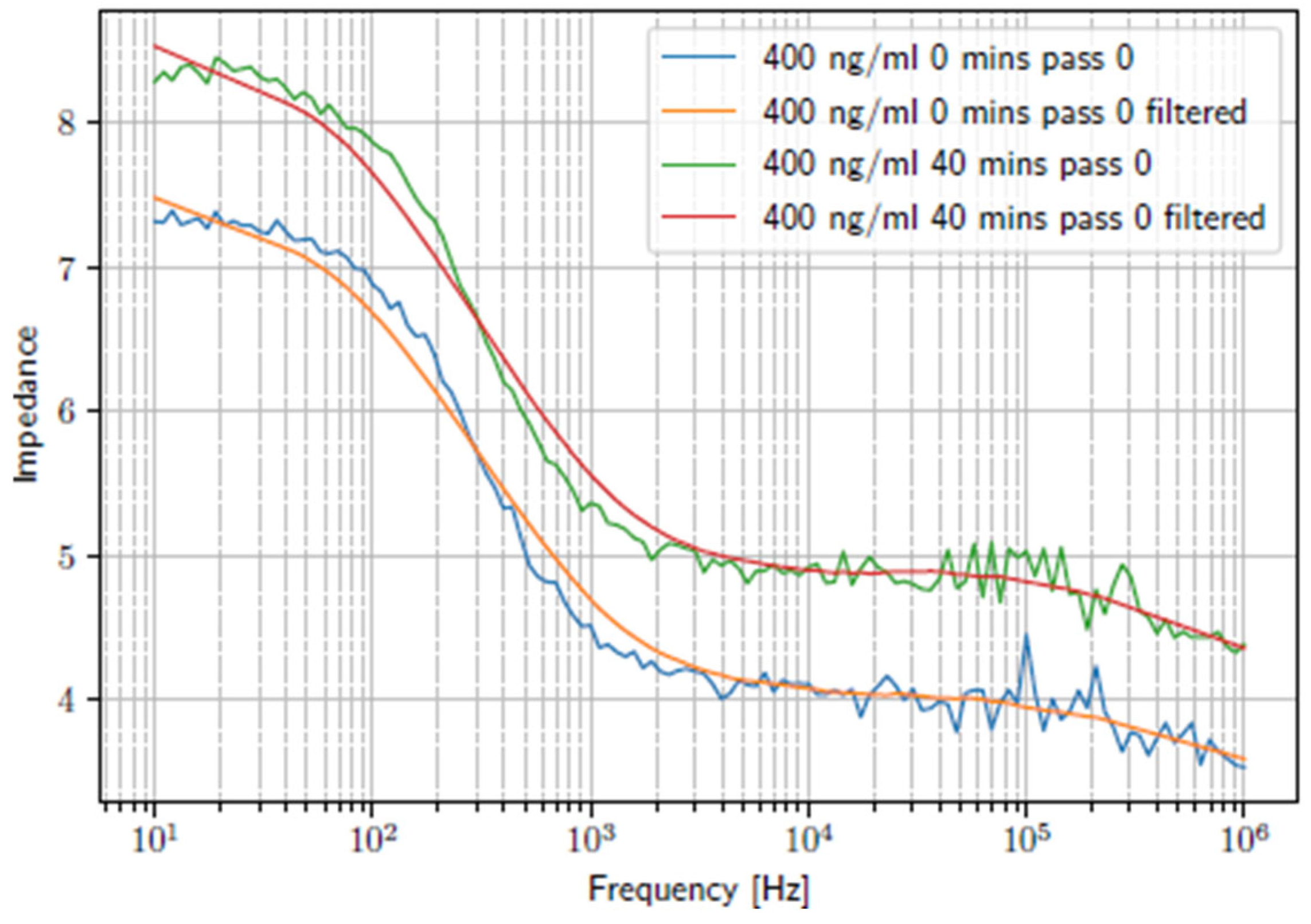
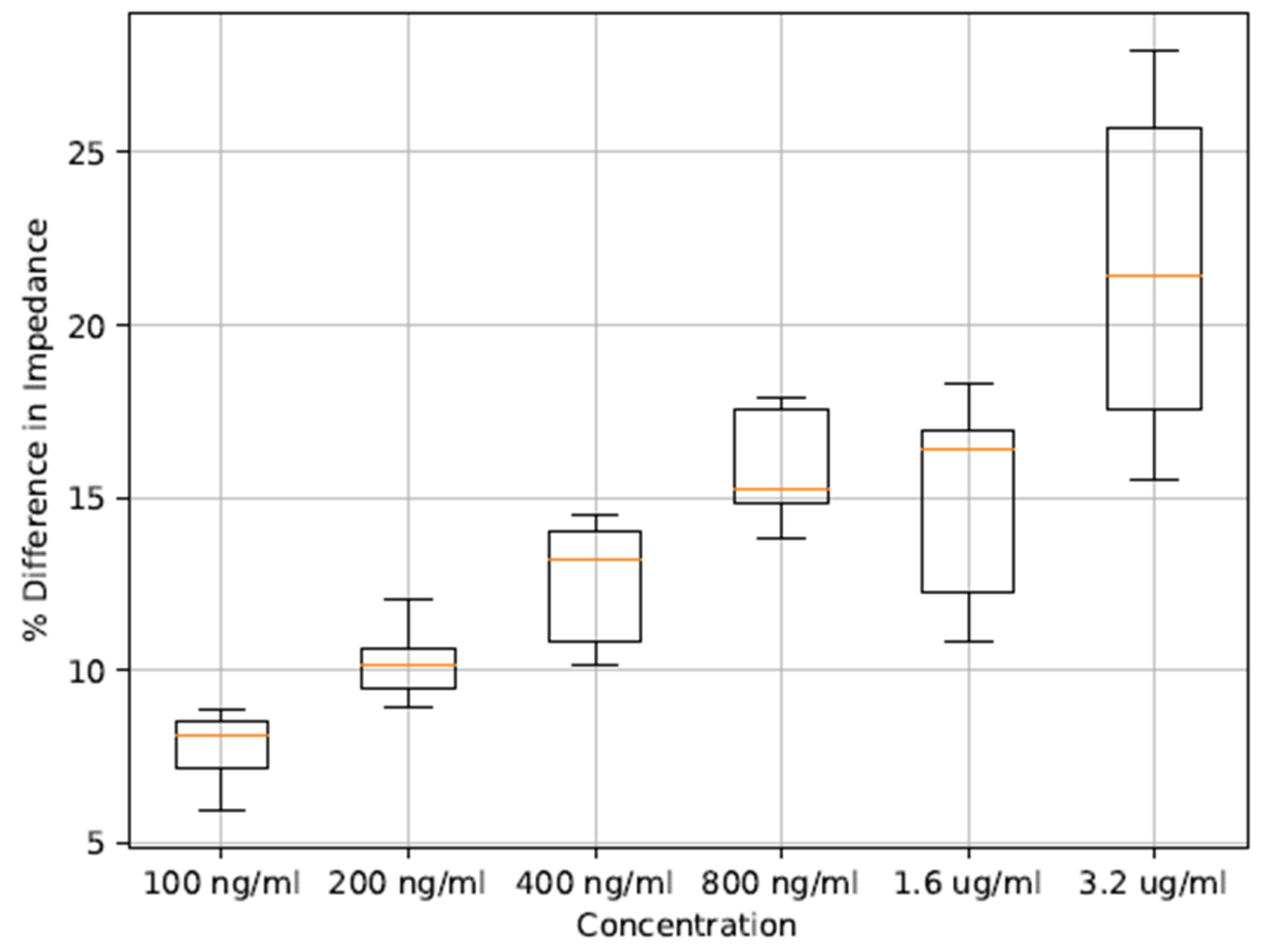
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; pp. 1–19.
- Dittrich, S.; Tadesse, B.T.; Moussy, F.; Chua, A.; Zorzet, A.; Tängdén, T.; Dolinger, D.L.; Page, A.; Crump, J.A.; D’Acremont, V.; et al. Target product profile for a diagnostic assay to differentiate between bacterial and non-bacterial infections and reduce antimicrobial overuse in resource-limited settings: An expert consensus. PLoS ONE 2016, 11, e0161721. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, S. Meeting of Experts on Biomarkers to Discriminate Bacterial from Other Infectious Causes of Acute Fever; World Health Organization: Geneva, Switzerland, 2015; pp. 5–23.
- Bernstein, D.; Coster, D.; Berliner, S.; Shapira, I.; Zeltser, D.; Rogowski, O.; Adler, A.; Halutz, O.; Levinson, T.; Ritter, O.; et al. C-reactive protein velocity discriminates between acute viral and bacterial infections in patients who present with relatively low CRP concentrations. BMC Infect. Dis. 2021, 21, 1210. [Google Scholar] [CrossRef] [PubMed]
- Levinson, T.; Wasserman, A. C-Reactive Protein Velocity (CRPv) as a New Biomarker for the Early Detection of Acute Infection/Inflammation. Int. J. Mol. Sci. 2022, 23, 8100. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Lloyd, A.M.; Perold, W.J.; Fourie, P.R. Electrospun Nano- and Microfiber Mesh-Based Transducer for Electrochemical Biosensing Applications. Eng. Proc. 2023, 58, 100. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloyd, A.; Perold, W.; Fourie, P. Development of a Nano- and Microfiber Mesh-Based Biosensor for the Rapid Quantification of Human C-Reactive Protein (CRP). Eng. Proc. 2025, 109, 15. https://doi.org/10.3390/engproc2025109015
Lloyd A, Perold W, Fourie P. Development of a Nano- and Microfiber Mesh-Based Biosensor for the Rapid Quantification of Human C-Reactive Protein (CRP). Engineering Proceedings. 2025; 109(1):15. https://doi.org/10.3390/engproc2025109015
Chicago/Turabian StyleLloyd, Alexander, Willem Perold, and Pieter Fourie. 2025. "Development of a Nano- and Microfiber Mesh-Based Biosensor for the Rapid Quantification of Human C-Reactive Protein (CRP)" Engineering Proceedings 109, no. 1: 15. https://doi.org/10.3390/engproc2025109015
APA StyleLloyd, A., Perold, W., & Fourie, P. (2025). Development of a Nano- and Microfiber Mesh-Based Biosensor for the Rapid Quantification of Human C-Reactive Protein (CRP). Engineering Proceedings, 109(1), 15. https://doi.org/10.3390/engproc2025109015





