Analysis of the Role of Temperature and Current Density in Hydrogen Production via Water Electrolysis: A Systematic Literature Review †
Abstract
1. Introduction
- RQ1: How does temperature affect the efficiency and hydrogen generation rate in various electrolyzers?
- RQ2: What is the relationship between current density and Faradaic efficiency?
- RQ3: What are the optimal combinations of temperature and current density for maximizing hydrogen output?
- RQ4: What are the current knowledge gaps and future research opportunities in this field?
2. Materials and Methods
2.1. Research Objectives and Questions
- RQ1: How does operating temperature affect the efficiency and hydrogen production rate in water electrolysis systems?
- RQ2: What is the effect of current density on Faradaic efficiency and system performance?
- RQ3: What combinations of temperature and current density yield optimal hydrogen production across different types of electrolyzers?
- RQ4: What are the current research gaps in this domain?
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
- Studies focused on hydrogen production using water electrolysis.
- Explicit investigation of temperature and/or current density as key variables.
- Research involving alkaline, PEM, or solid oxide electrolyzer systems.
- Experimental or simulation-based results.
- Studies unrelated to hydrogen production or involving biological, photocatalytic, or chemical hydrogen generation routes.
- Reviews without primary data.
- Papers lacking full-text access or written in languages other than English.
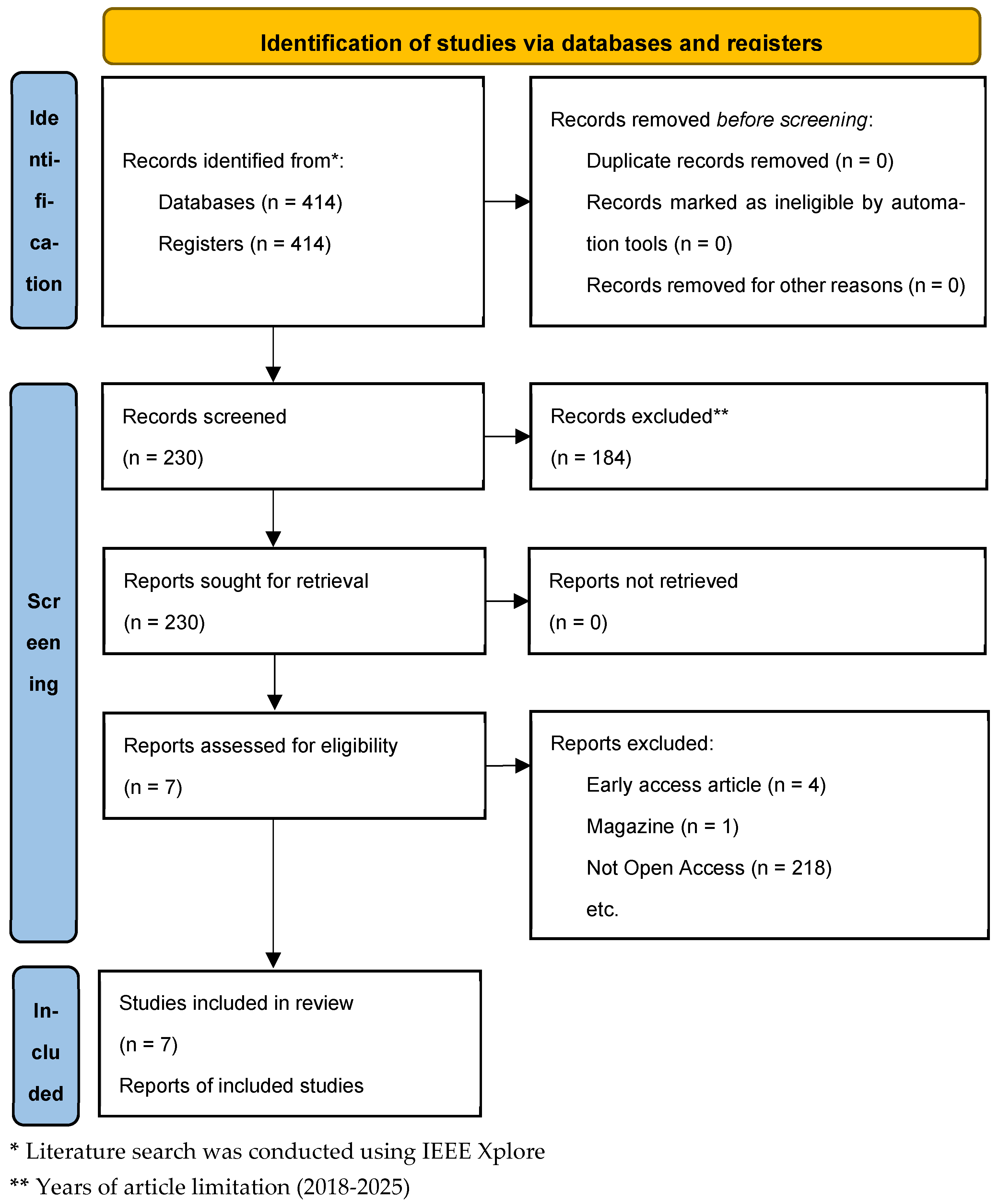
2.4. Study Selection and Screening Process
- Identification—Initial database search retrieved 414 records.
- Screening—After years of article limitation, 230 titles and abstracts were screened.
- Eligibility—7 full-text articles were reviewed for relevance and quality.
- Inclusion—7 high-quality studies were selected for final data extraction and analysis.
2.5. Data Extraction
- Bibliographic data (author, year, journal);
- Type of electrolyzer (AEL, PEM, SOEC);
- Temperature and current density values;
- Hydrogen production rate (mol/s or L/min);
- Faradaic and energy efficiency (%);
- Key findings and conclusions.
2.6. Quality Assessment
- Methodological transparency;
- Clarity of parameter reporting;
- Experimental or simulation validity;
- Relevance to the research questions.
3. Results
3.1. PRISMA Flow Diagram
- Early access or non-peer-reviewed format (4 articles);
- Non-scientific sources (e.g., magazines) (1 articles);
- Paywalled or inaccessible articles (218 articles).
3.2. Summary of Extracted Data
- Mao et al. (2024) developed a second-order equivalent circuit model for PEM electrolyzers, incorporating thermal effects [14].
- Li et al. (2024) analyzed solid-state transformer architectures tailored for high-capacity electrolyzer loads [15].
- Xing et al. (2018) reviewed modeling strategies across all electrolyzer types, with emphasis on system dynamics and operating envelopes [16].
- Liu et al. (2024) introduced optimal capacity planning that integrates SOEC in multi-vector energy systems [17].
- Zeng et al. (2024) applied stochastic scheduling to model seasonal hydrogen storage and conversion via PtG routes [18].
- Zhong et al. (2023) reviewed recent technological advancements and challenges in electrolysis, highlighting catalyst development and system-level optimization [19].
- Giuliani et al. (2023) assessed the techno-economic viability of power-to-hydrogen-to-power (P2H2P) schemes, especially comparing intermittent versus continuous hydrogen production from nuclear fusion [20].
4. Discussion
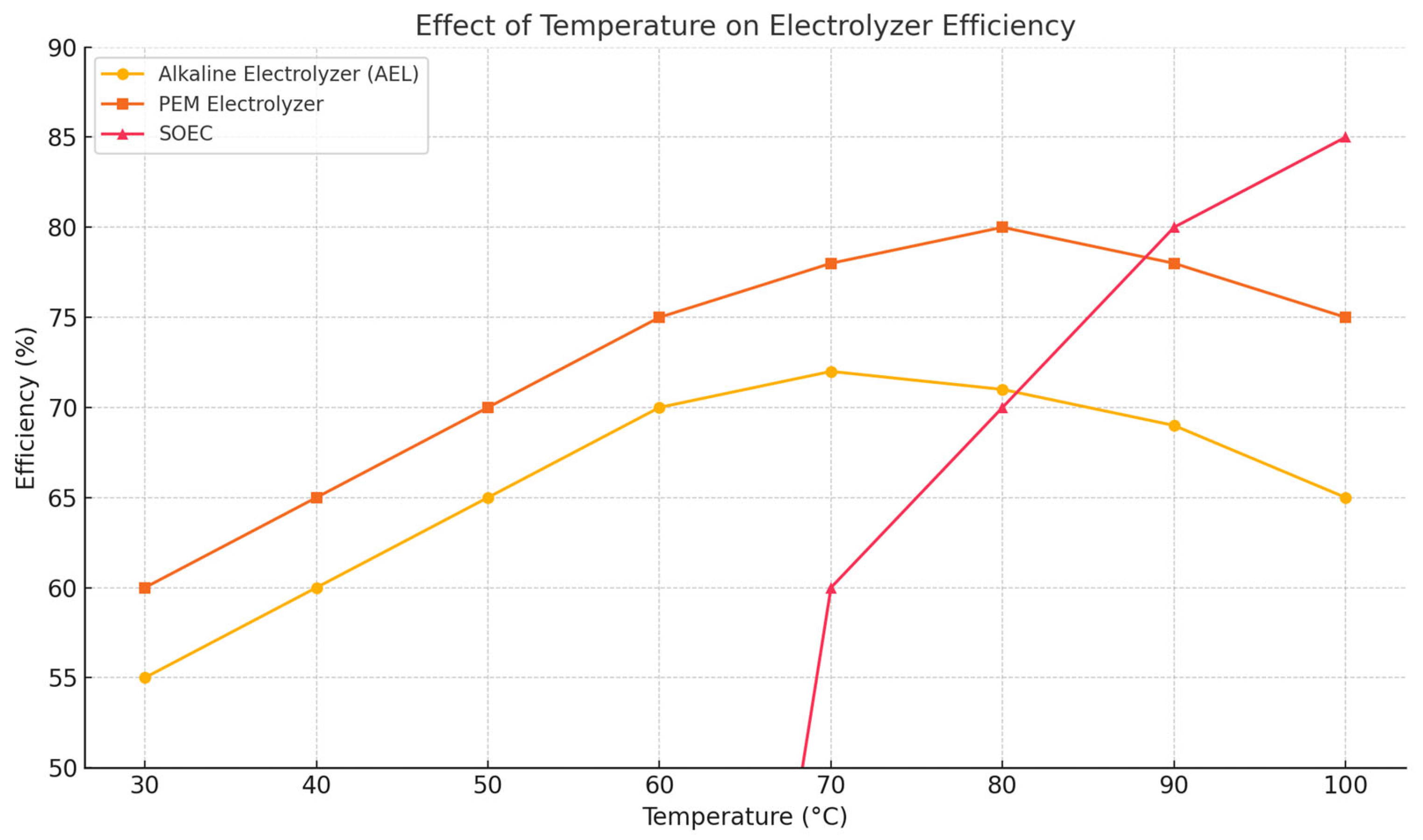
4.1. Impact of Temperature on Electrolysis Performance
- The Alkaline Electrolyzer (AEL) shows optimum efficiency at around 60–70 °C.
- PEM electrolyzers perform best in the 70–80 °C range.
- SOEC works efficiently only at high temperatures (simulated from 70 °C and above in this graph).
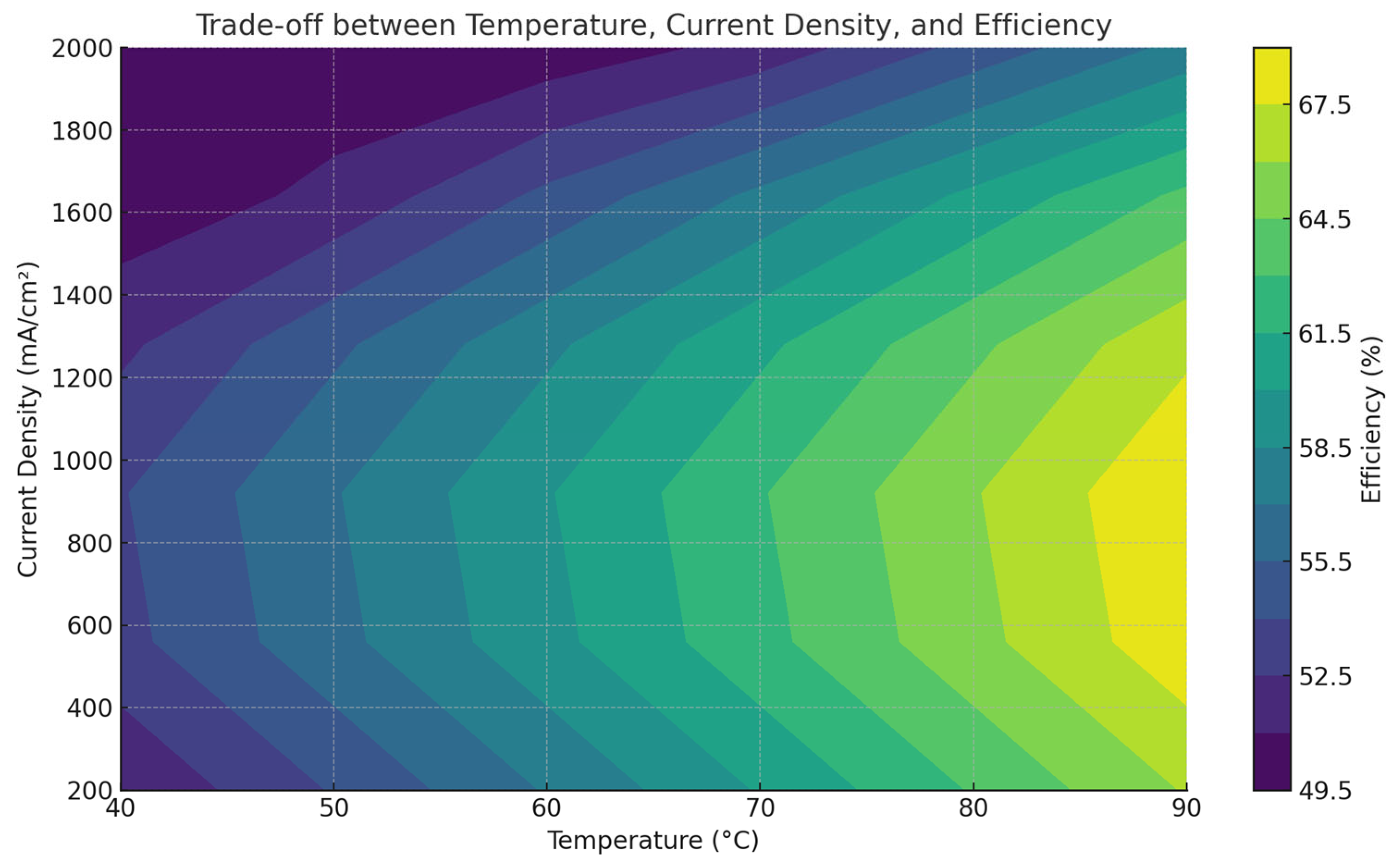
4.2. Effect of Current Density
4.3. Combined Effects and Optimal Operating Points
4.4. System-Level Perspectives and Integration Challenges
4.5. Research Gaps and Future Directions
- (a)
- Dynamic and transient behavior modeling: Most models assume steady-state or quasi-steady operation. Future studies should explore the dynamic response of electrolyzers under real-time load-following scenarios.
- (b)
- Thermal–electrical co-optimization: Advanced control algorithms that jointly manage temperature and current to prevent hotspots or material fatigue are needed.
- (c)
- Scalable system integration: Studies should address the practical challenges of integrating MW-scale electrolyzers with renewable grids, including power electronics, storage sizing, and control logic.
- (d)
- Lifecycle and degradation studies: Long-term performance data under different operating regimes are still scarce and critical for techno-economic viability.
- (e)
- Socio-economic evaluations: Beyond technical feasibility, studies like Giuliani et al. (2023) show the importance of evaluating system cost and affordability at the national or regional scale [20].
5. Conclusions
- RQ1: How does operating temperature affect the efficiency and hydrogen generation rate in water electrolysis systems?Higher temperatures generally improve reaction kinetics and lower cell voltage, especially in PEM and SOEC systems. However, they also introduce material degradation risks, underscoring the need for thermal control and material innovations.
- RQ2: What is the effect of current density on Faradaic efficiency and system performance?Increased current density enhances hydrogen output but may reduce Faradaic efficiency and accelerate electrode degradation, particularly in AEL and PEM systems. An optimal operational window is required for balancing yield and stability.
- RQ3: What combinations of temperature and current density yield optimal hydrogen production?PEMELs operate optimally at 60–80 °C and 500–1000 mA/cm2; AELs operate optimally at 50–60 °C and 200–500 mA/cm2; SOECs exceed 750 °C with lower current loads. Optimal performance depends on electrolyzer type, thermal environment, and system integration level.
- RQ4: What are the current research gaps and future opportunities?Major gaps include dynamic behavior modeling under fluctuating loads, co-optimization of thermal–electrical control, and long-term durability assessments. System-level challenges such as storage integration, grid interfacing, and economic feasibility also require deeper exploration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guilbert, D.; Vitale, G. Hydrogen as a Clean and Sustainable Energy Vector for Global Transition from Fossil-Based to Zero-Carbon. Clean Technol. 2021, 3, 881–909. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Jahirul, M.I.; Sattar, M.A.; Hassan, N.M.S. Hydrogen Horizons: Navigating Challenges and Opportunities for a Sustainable Energy Future. In Proceedings of the 2023 International Conference on Sustainable Technology and Engineering (i-COSTE), Nadi, Fiji, 4–6 December 2023; pp. 1–7. [Google Scholar] [CrossRef]
- Paidar, M.; Bouzek, K. Water Electrolysis as an Environmentally Friendly Source of Hydrogen. In Hydrogen Production and Energy Transition; De Gruyter: Berlin, Germany, 2021; pp. 331–358. [Google Scholar] [CrossRef]
- Quang, D.V.; Milani, D.; Abu Zahra, M. A Review of Potential Routes to Zero and Negative Emission Technologies via the Integration of Renewable Energies with CO2 Capture Processes. Int. J. Greenh. Gas Control 2023, 124, 103862. [Google Scholar] [CrossRef]
- Amina, S.; Laila, A.; Nihel, C.; Fadi, A. Effect of Temperature on the Performance of Proton Exchange Membrane Water Electrolysis. In Proceedings of the 2025 15th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 2–4 February 2025; pp. 1–4. [Google Scholar] [CrossRef]
- Lohmann-Richters, F.P.; Renz, S.; Lehnert, W.; Müller, M.; Carmo, M. Review—Challenges and Opportunities for Increased Current Density in Alkaline Electrolysis by Increasing the Operating Temperature. J. Electrochem. Soc. 2021, 168, 114501. [Google Scholar] [CrossRef]
- Hu, K.; Fang, J.; Ai, X.; Huang, D.; Zhong, Z.; Yang, X.; Wang, L. Comparative Study of Alkaline Water Electrolysis, Proton Exchange Membrane Water Electrolysis and Solid Oxide Electrolysis through Multiphysics Modeling. Appl. Energy 2022, 312, 118788. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current Status, Research Trends, and Challenges in Water Electrolysis Science and Technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Sebbahi, S.; Nabil, N.; Alaoui-Belghiti, A.; Laasri, S.; Rachidi, S.; Hajjaji, A. Assessment of the Three Most Developed Water Electrolysis Technologies: Alkaline Water Electrolysis, Proton Exchange Membrane and Solid-Oxide Electrolysis. Mater. Today Proc. 2022, 66, 140–145. [Google Scholar] [CrossRef]
- Şahin, M.E. An Overview of Different Water Electrolyzer Types for Hydrogen Production. Energies 2024, 17, 4944. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kalauni, K.; Pahwa, R. Water Electrolysis Technologies and Their Modeling Approaches: A Comprehensive Review. Eng 2025, 6, 81. [Google Scholar] [CrossRef]
- Nguyen, E.; Olivier, P.; Pera, M.-C.; Pahon, E.; Roche, R. Impacts of Intermittency on Low-Temperature Electrolysis Technologies: A Comprehensive Review. Int. J. Hydrogen Energy 2024, 70, 474–492. [Google Scholar] [CrossRef]
- Arsad, S.R.; Arsad, A.Z.; Ker, P.J.; Hannan, M.A.; Tang, S.G.; Goh, S.M.; Mahlia, T.M.I. Recent Advancement in Water Electrolysis for Hydrogen Production: A Comprehensive Bibliometric Analysis and Technology Updates. Int. J. Hydrogen Energy 2024, 60, 780–801. [Google Scholar] [CrossRef]
- Mao, X.; Tian, Y.; Yang, A.; Zhang, G. Identification of Equivalent Circuit Parameters for Proton Exchange Membrane (PEM) Electrolyzer Engineering Models. IEEE Access 2024, 12, 15509–15524. [Google Scholar] [CrossRef]
- Li, Z.; Mirzadarani, R.; Niasar, M.G.; Itraj, M.; van Lieshout, L.; Bauer, P.; Qin, Z. Medium-Voltage Solid-State Transformer Design for Large-Scale H2 Electrolyzers. IEEE Open J. Power Electron. 2024, 5, 936–955. [Google Scholar] [CrossRef]
- Xing, X.; Lin, J.; Song, Y.; Zhou, Y.; Mu, S.; Hu, Q. Modeling and Operation of the Power-to-Gas System for Renewables Integration: A Review. CSEE J. Power Energy Syst. 2018, 4, 168–178. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Wang, Y.; Wu, L. Optimal Capacity Configuration of a Low-Carbon Energy System Considering Carbon Capture Technology and Hydrogen-Diversified Utilization under Multiple Operational Scenarios. IEEE Access 2024, 12, 85–112. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, C.; Liao, M.; Luo, Y.; Dong, X. Optimal Stochastic Scheduling Strategy of Multi-Vector Energy Complex Integrated with Full-Blown Power-to-Biomethane Model. J. Mod. Power Syst. Clean Energy 2024, 12, 863–873. [Google Scholar] [CrossRef]
- Zhong, Z.; Fang, J.; Hu, K.; Huang, D.; Ai, X.; Yang, X.; Wen, J.; Pan, Y.; Cheng, S. Power-to-Hydrogen by Electrolysis in Carbon Neutrality: Technology Overview and Future Development. CSEE J. Power Energy Syst. 2023, 9, 1266–1283. [Google Scholar] [CrossRef]
- Giuliani, U.; Agostini, M.; Bustreo, C.; Zollino, G. The Fusion to Hydrogen Option in a Carbon Free Energy System. IEEE Access 2023, 11, 131178–131190. [Google Scholar] [CrossRef]
| Author (Year) | Type of Electrolyzer | Temp (°C) | Current Density (mA/cm2) | H2 Prod. Rate | Faradaic Efficiency (%) | Key Findings |
|---|---|---|---|---|---|---|
| Mao et al. (2024) [14] | PEMEL | 25–80 * | Variable (not fixed) | Model-based | N/A (focus on modeling) | Developed an enhanced second-order RC model accounting for heat losses; useful for control systems. |
| Li et al. (2024) [15] | Not electrolyzer but SST interface for H2 system | N/A | N/A | N/A | N/A | Proposed and benchmarked three SST topologies (MMC, MMR, ISOP) to power large-scale H2 electrolyzers. |
| Xing et al. (2018) [16] | Alkaline, PEM, SOEC | 60–1000 (varied) | <0.5–<2 A/cm2 | Qualitative/comparative | 55–95% (system level) | Comprehensive review of P2G modeling and electrolyzer types; discusses T and I effect qualitatively. |
| Liu et al. (2024) [17] | SOEC (integrated) | High-temp SOEC | Implied through model | Modeled via ζsoec | 90% (SOEC) | Constructs a low-carbon energy system integrating SOEC for hydrogen production in multiple use cases. |
| Zeng et al. (2024) [18] | SOEC + Methanation | ~600 (SOEC) | Not explicitly stated | ηel modeled thermodynamically | Modeled via ηel | Develops a stochastic scheduling model of MECs with detailed modeling of electrolysis and PtM systems. |
| Zhong et al. (2023) [19] | AEL, PEM, SOEC, AEM | AEL: 60; SOEC: ~800 | AEL: 2000–4000; PEM: 10,000–20,000 | System-level estimation (qualitative) | 60–90% (estimated) | Comprehensive multidisciplinary review of PtHE tech, emphasizing multiphysics coupling, catalyst, and strategy gaps. |
| Giuliani et al. (2023) [20] | PEM (modeled via P2H2P) | Not specified (modeled) | Not specified (modeled) | System simulation (P2H2P) | Not explicitly stated | Evaluates economic feasibility of hydrogen production from fusion energy and overgeneration; identifies storage cost bottlenecks. |
| Author (Year) | Q1: Method Clarity (1–5) | Q2: Parameter Detail (1–5) | Q3: Experimental Validity (1–5) | Q4: Relevance to RQs (1–5) | Total Score (Max 20) | Included? |
|---|---|---|---|---|---|---|
| Mao et al. (2024) [14] | 5 | 4 | 4 | 5 | 18 | Yes |
| Li et al. (2024) [15] | 5 | 4 | 3 | 3 | 15 | Yes |
| Xing et al. (2018) [16] | 5 | 4 | 3 | 5 | 17 | Yes |
| Liu et al. (2024) [17] | 5 | 4 | 4 | 4 | 17 | Yes |
| Zeng et al. (2024) [18] | 5 | 5 | 4 | 5 | 19 | Yes |
| Zhong et al. (2023) [19] | 5 | 5 | 4 | 5 | 19 | Yes |
| Giuliani et al. (2023) [20] | 5 | 3 | 3 | 4 | 15 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narputro, P.; Effendi, P.; Akbar, I.M.; Rahman, S. Analysis of the Role of Temperature and Current Density in Hydrogen Production via Water Electrolysis: A Systematic Literature Review. Eng. Proc. 2025, 107, 23. https://doi.org/10.3390/engproc2025107023
Narputro P, Effendi P, Akbar IM, Rahman S. Analysis of the Role of Temperature and Current Density in Hydrogen Production via Water Electrolysis: A Systematic Literature Review. Engineering Proceedings. 2025; 107(1):23. https://doi.org/10.3390/engproc2025107023
Chicago/Turabian StyleNarputro, Panji, Prastiyo Effendi, Iqbal Maulana Akbar, and Saefur Rahman. 2025. "Analysis of the Role of Temperature and Current Density in Hydrogen Production via Water Electrolysis: A Systematic Literature Review" Engineering Proceedings 107, no. 1: 23. https://doi.org/10.3390/engproc2025107023
APA StyleNarputro, P., Effendi, P., Akbar, I. M., & Rahman, S. (2025). Analysis of the Role of Temperature and Current Density in Hydrogen Production via Water Electrolysis: A Systematic Literature Review. Engineering Proceedings, 107(1), 23. https://doi.org/10.3390/engproc2025107023






