Prediction of Asthma Disease Using Machine Learning Algorithm †
Abstract
1. Introduction
2. Literature Review
3. Methodology
3.1. Boxplot
3.2. Dataset Description
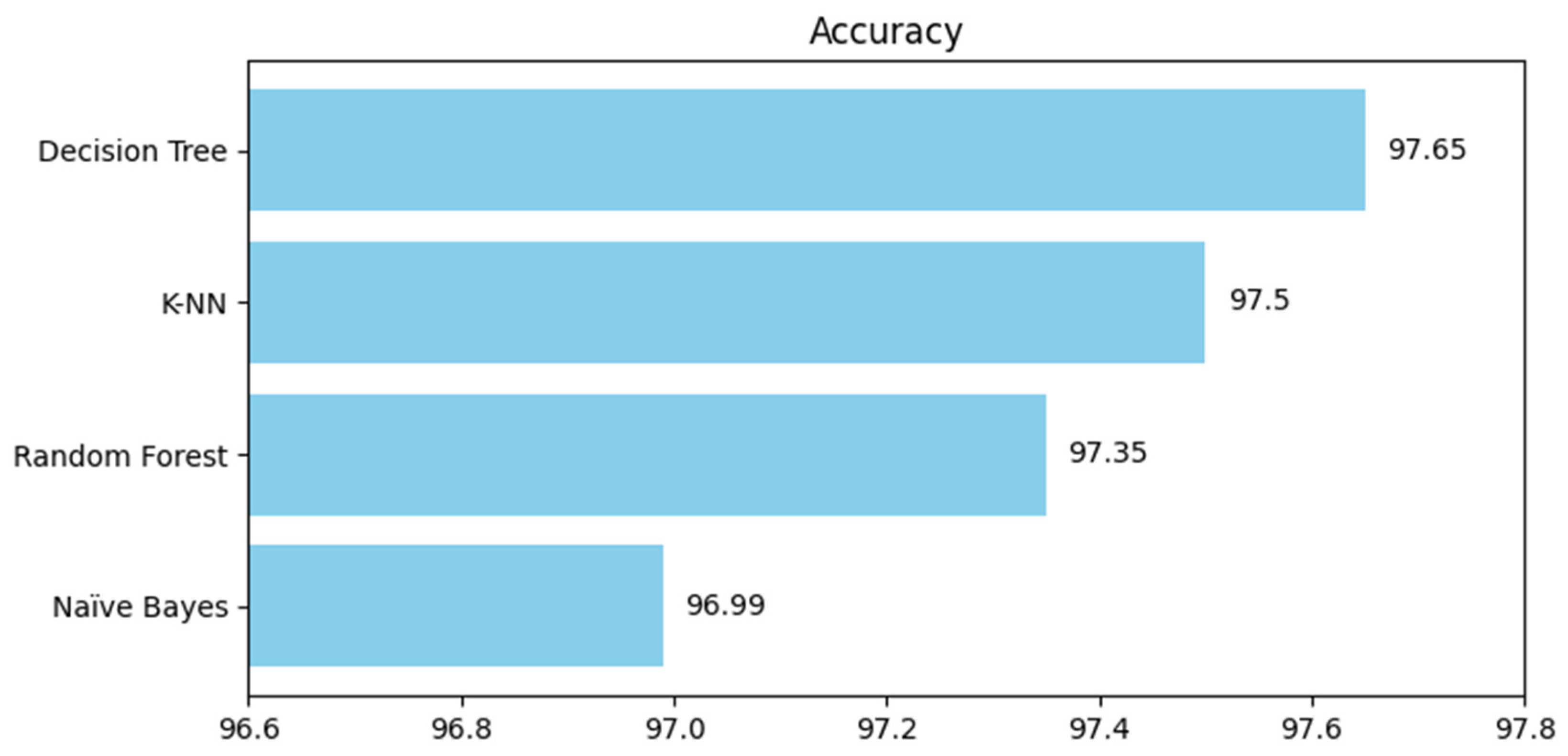
4. Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavitha, K.S.; Monisha, M.; Nischitha, M.; Nisha, M.; Raksitha, A. Asthma Prediction and Monitoring. Indian J. Allergy Asthma Immunol. 2023, 37, 33–36. [Google Scholar] [CrossRef]
- Fontanella, S.; Cucco, A.; Custovic, A. Machine Learning in Asthma Research: Moving Toward a More Integrated Approach. Expert Rev. Respir. Med. 2021, 15, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.S.; Shankar, N.; Kim, D.; Song, D.J.; Seo, S.; Panahi, I.M.; Tamil, L. Machine Learning-Based Asthma Risk Prediction Using IoT and Smartphone Applications. IEEE Access 2021, 9, 118708–118715. [Google Scholar] [CrossRef]
- Spathis, D.; Vlamos, P. Diagnosing Asthma and Chronic Obstructive Pulmonary Disease with Machine Learning. Health Inform. J. 2019, 25, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.C.H.; Pinnock, H.; Wilson, A.M.; Shah, S.A. Application of Machine Learning Algorithms for Asthma Management with mHealth: A Clinical Review. J. Asthma Allergy 2022, 2022, 855–873. [Google Scholar] [CrossRef]
- Kothalawala, D.M.; Murray, C.S.; Simpson, A.; Custovic, A.; Tapper, W.J.; Arshad, S.H.; Holloway, J.W.; Rezwan, F.I.; STELAR/UNICORN Investigators. Development of Childhood Asthma Prediction Models Using Machine Learning Approaches. Clin. Transl. Allergy 2021, 11, e12076. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Chen, W.; Cheng, L.; Wang, Q.; Han, F.; Cui, Y. Diagnosis of Asthma Based on Routine Blood Biomarkers Using Machine Learning. Comput. Intell. Neurosci. 2020, 2020, 8841002. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.J.; Yang, M.R.; Hwang, B.J. A Sustainable Approach to Asthma Diagnosis: Classification with Data Augmentation, Feature Selection, and Boosting Algorithm. Diagnostics 2024, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Gaudillo, J.; Rodriguez, J.J.R.; Nazareno, A.; Baltazar, L.R.; Vilela, J.; Bulalacao, R.; Domingo, M.; Albia, J. Machine Learning Approach to Single Nucleotide Polymorphism-Based Asthma Prediction. PLoS ONE 2019, 14, e0225574. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Jena, J.J.; Gourisaria, M.K. Predictive Modeling for Asthma Disease Detection: A Comparative Study of Machine Learning Algorithms. 2024. Available online: https://hal.science/hal-04731433v1 (accessed on 1 January 2025).
- Molfino, N.A.; Turcatel, G.; Riskin, D. Machine Learning Approaches to Predict Asthma Exacerbations: A Narrative Review. Adv. Ther. 2024, 41, 534–552. [Google Scholar] [CrossRef] [PubMed]
- Turcatel, G.; Xiao, Y.; Caveney, S.; Gnacadja, G.; Kim, J.; Molfino, N.A. Predicting Asthma Exacerbations Using Machine Learning Models. Adv. Ther. 2024, 42, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chen, W.; Jia, X.; Jia, Y.; Liu, C. Machine Learning for Prediction of Asthma Exacerbations Among Asthmatic Patients: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2023, 23, 278. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, M.C.; Marinho, S.; Simpson, A.; Custovic, A.; Buchan, I.E. Predicting Phenotypes of Asthma and Eczema with Machine Learning. BMC Med. Genom. 2014, 7, S7. [Google Scholar] [CrossRef] [PubMed]
- Chatzimichail, E.; Paraskakis, E.; Sitzimi, M.; Rigas, A. An intelligent system approach for asthma prediction in symptomatic preschool children. Comput. Math. Methods Med. 2013, 2013, 240182. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Abhadiomhen, S.E.; Zhou, D.; Shen, X.J.; Shi, L.; Cui, Y. Asthma Prediction via Affinity Graph Enhanced Classifier: A Machine Learning Approach Based on Routine Blood Biomarkers. J. Transl. Med. 2024, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Airehrour, D.; Gutierrez, J.; Kumar Ray, S. GradeTrust: A secure trust based routing protocol for MANETs. In Proceedings of the 25th International Telecommunication Networks and Applications Conference (ITNAC), Sydney, NSW, Australia, 18–20 November 2015; pp. 65–70. [Google Scholar] [CrossRef]
- Diwaker, C.; Tomar, P.; Solanki, A.; Nayyar, A.; Jhanjhi, N.Z.; Abdullah, A.; Supramaniam, M. A New Model for Predicting Component- Based Software Reliability Using Soft Computing. IEEE Access 2019, 7, 147191–147203. [Google Scholar] [CrossRef]
- Kok, S.H.; Abdullah, A.; Jhanjhi, N.Z.; Supramaniam, M. A review of intrusion detection system using machine learning approach. Int. J. Eng. Res. Technol. 2019, 12, 8–15. [Google Scholar]


| Attribute 1 | Attribute 2 |
|---|---|
| Patient ID | Pollution Exposure |
| Age | Pollen Exposure |
| Gender | Dust Exposure |
| Ethnicity | Pet Allergy |
| Education Level | Family History of Asthma |
| BMI | History of Allergies |
| Smoking | Eczema |
| Physical Activity | Hay Fever |
| Diet Quality | Sleep Quality |
| Gastroesophageal Symptoms | Reflux |
| Lung Function FEV1 | Lung Function FVC |
| Wheezing | Shortness of Breath |
| Chest Tightness | Coughing |
| Nighttime Symptoms | Exercise-Induced Diagnosis |
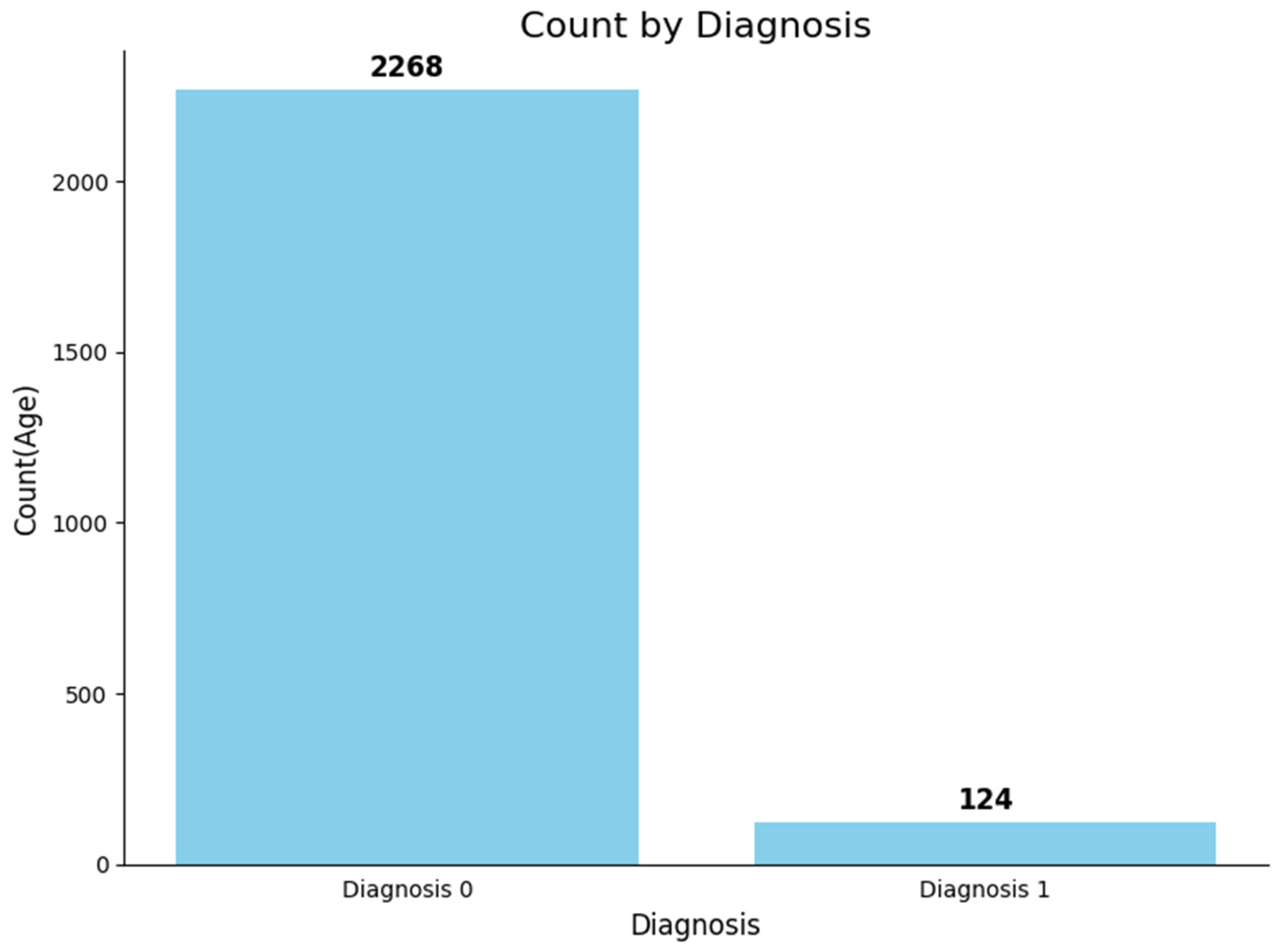
| Class | Count | Percentage | Precision | Recall |
|---|---|---|---|---|
| 0 | 2268 | 94.81% | 99.69% | 95.59% |
| 1 | 124 | 5.18% | 95.76% | 99.71% |
| True 0 | True 1 | Class Precision | Class Recall | |
|---|---|---|---|---|
| 0 | 650 | 2 | 99.69% | 95.59% |
| 1 | 30 | 678 | 95.76% | 99.71% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahab; Hussain, M.; Parwati, L.S. Prediction of Asthma Disease Using Machine Learning Algorithm. Eng. Proc. 2025, 107, 115. https://doi.org/10.3390/engproc2025107115
Zahab, Hussain M, Parwati LS. Prediction of Asthma Disease Using Machine Learning Algorithm. Engineering Proceedings. 2025; 107(1):115. https://doi.org/10.3390/engproc2025107115
Chicago/Turabian StyleZahab, Manzoor Hussain, and Lusiana Sani Parwati. 2025. "Prediction of Asthma Disease Using Machine Learning Algorithm" Engineering Proceedings 107, no. 1: 115. https://doi.org/10.3390/engproc2025107115
APA StyleZahab, Hussain, M., & Parwati, L. S. (2025). Prediction of Asthma Disease Using Machine Learning Algorithm. Engineering Proceedings, 107(1), 115. https://doi.org/10.3390/engproc2025107115





