Abstract
Optical biosensors have emerged as a transformative technology for food safety monitoring. These devices combine biorecognition molecules with advanced optical transducers, enabling the detection of a wide array of food contaminants, including pathogens, toxins, pesticides, and antibiotic residues. This review comprehensively explores the principles, advancements, applications, and future trends of optical biosensors in ensuring food safety. The key advantages of optical biosensors, such as high sensitivity to trace contaminants, fast response times, and portability, make them an attractive alternative to traditional analytical methods. Types of optical biosensors discussed include surface plasmon resonance (SPR), interferometric, fluorescence and chemiluminescence, and colorimetric biosensors. SPR biosensors stand out for their real-time, label-free analysis of foodborne pathogens and contaminants, while fluorescence and chemiluminescence biosensors offer exceptional sensitivity for detecting low levels of toxins. Interferometric and colorimetric biosensors, characterized by their portability and visual signal output, are well-suited for field-based applications. Biosensors have proven invaluable in monitoring heavy metals, pesticide residues, and antibiotic contaminants, ensuring compliance with stringent food safety standards. The integration of nanotechnology has further enhanced the performance of optical biosensors, with nanomaterials such as quantum dots and nanoparticles enabling ultra-sensitive detection and signal amplification. Optical biosensors represent a vital advancement in the field of food safety, addressing critical public health concerns through their rapid and reliable detection capabilities. Continued interdisciplinary efforts in nanotechnology, material science, and device engineering are poised to further expand their applications, making them indispensable tools for safeguarding global food supply chains.
1. Introduction
Optical biosensors have emerged as a vital tool in modern food safety monitoring, offering a combination of high sensitivity, specificity, and real-time detection capabilities. These sensors operate by converting a biological recognition event—such as antigen–antibody binding or nucleic acid hybridization—into an optical signal, which is then measured using methods such as fluorescence, chemiluminescence, absorbance, or SPR [1]. Their ability to rapidly and accurately identify biological and chemical contaminants makes them exceptionally well-suited for ensuring the safety and quality of food products at every stage of the supply chain. One of the key advantages of optical biosensors lies in their sensitivity and specificity, which allows for the detection of trace levels of harmful substances such as pathogens, toxins, and pesticide residues [2,3]. These sensors also offer rapid analysis, enabling real-time monitoring that can prevent the distribution of contaminated food before it reaches consumers [4,5]. Moreover, their portability and user-friendly design facilitate on-site testing in agricultural fields, food processing plants, and marketplaces without requiring specialized lab infrastructure [6,7]. Compared to traditional analytical methods, optical biosensors significantly reduce the cost, complexity, and turnaround time of food safety testing, making them particularly valuable in low-resource settings [8]. Several types of optical biosensors have found success in food applications. SPR sensors are among the most widely used, offering label-free, real-time monitoring with high sensitivity. They are effective for detecting pesticide residues, toxins, and foodborne pathogens [2,4]. Fluorescence and chemiluminescence-based sensors, on the other hand, offer amplified optical signals and are frequently used to detect aflatoxins, mycotoxins, and other low-abundance analytes in complex food matrices [9,10]. Interferometric biosensors provide a powerful approach for multiplexed detection, enabling the simultaneous monitoring of multiple contaminants with minimal cross-talk, making them ideal for comprehensive safety evaluations [11]. In terms of real-world applications, optical biosensors are routinely used for the detection of microbial pathogens such as Salmonella, Listeria monocytogenes, and E. coli in food and water samples [3,12]. They are also employed in monitoring chemical hazards, including residues of heavy metals, antibiotics, and illicit food additives that can pose serious health risks [8]. Additionally, optical biosensors are playing an increasing role in quality control, helping to monitor freshness, storage conditions, and shelf life indicators in processed and packaged food [7,13]. Despite their numerous advantages, optical biosensors face several challenges. Non-specific interactions between the sensor surface and sample components can compromise detection accuracy, particularly in complex food matrices. Addressing this requires advanced surface chemistry strategies and robust sensor coatings [14]. Moreover, integrating nanomaterials into optical biosensor platforms has emerged as a promising solution to enhance sensitivity, stability, and signal amplification. Nanostructures such as quantum dots, gold nanoparticles, and carbon-based nanomaterials improve light–matter interactions and facilitate stronger signal outputs [10]. Another key area of development is miniaturization—the design of compact, portable, and field-deployable biosensors remains a priority for real-time testing at critical control points throughout the food production and distribution chain [5]. Regulatory and standardization challenges remain a barrier to widespread commercialization. Despite strong laboratory performance, many optical biosensors still lack validated protocols and official approval for use in routine regulatory testing. Bridging this gap will require collaborative efforts among scientists, manufacturers, and policymakers to establish performance benchmarks, ensure quality control, and support the translation of laboratory prototypes into robust commercial tools [2,13].
Molecular and Biochemical Mechanisms of Optical Biosensors
To understand the underlying mechanisms and reactivity pathways of optical biosensors, it is essential to understand their molecular, biochemical, and optical interactions. Optical biosensors are analytical devices that combine a biorecognition element with an optical transducer to detect and analyze biological molecules or chemical substances. At their core, optical biosensors consist of a biorecognition element immobilized on a sensor surface and an optical transducer that converts biological interactions into measurable optical signals [15]. The biorecognition component can include antibodies, enzymes, or nucleic acids, each of which engages in highly specific molecular interactions with a target analyte, such as an antigen, substrate, or complementary nucleic acid strand [16,17].
At the biochemical level, antigen–antibody binding remains one of the most widely used pathways. When an antigen binds to its antibody immobilized on the biosensor surface, the interaction alters local optical properties, producing a measurable signal [18]. Similarly, nucleic acid biosensors rely on the hybridization of complementary DNA or RNA strands, where duplex formation produces a detectable change in fluorescence or refractive index [19]. Enzyme-based biosensors function through enzyme–substrate reactions, in which enzymatic activity either generates or consumes optically active molecules, leading to fluorescence, luminescence, or color changes [20]. Effective immobilization techniques such as covalent bonding, adsorption, or polymer entrapment are essential to preserve the stability and activity of these recognition molecules [21].
The optical mechanisms underlying detection are equally crucial. Surface plasmon resonance (SPR) biosensors measure changes in refractive index at a metal–dielectric interface when analytes bind to immobilized recognition elements, shifting the resonance angle or wavelength [16,17]. Fluorescence- and luminescence-based sensors employ fluorescent tags or luminescent molecules to generate measurable emission changes in response to analyte binding [22]. Interferometric sensors detect phase shifts in light waves as a function of analyte binding, offering high sensitivity to mass or refractive index variations [23,24].
Taken together, these reactivity pathways and optical mechanisms illustrate that optical biosensors operate not merely as detection devices, but as carefully engineered systems where biological recognition events are tightly coupled with photonic signal generation. Through antibody–antigen binding, nucleic acid hybridization, and enzyme–substrate catalysis, these biosensors translate biochemical specificity into quantifiable optical signals [15,16,23]. As such, understanding these fundamental processes is critical for advancing biosensor design, improving sensitivity, and ensuring their reliability in real-world applications.
2. Types of Optical Biosensors and Their Applications in the Food Industry
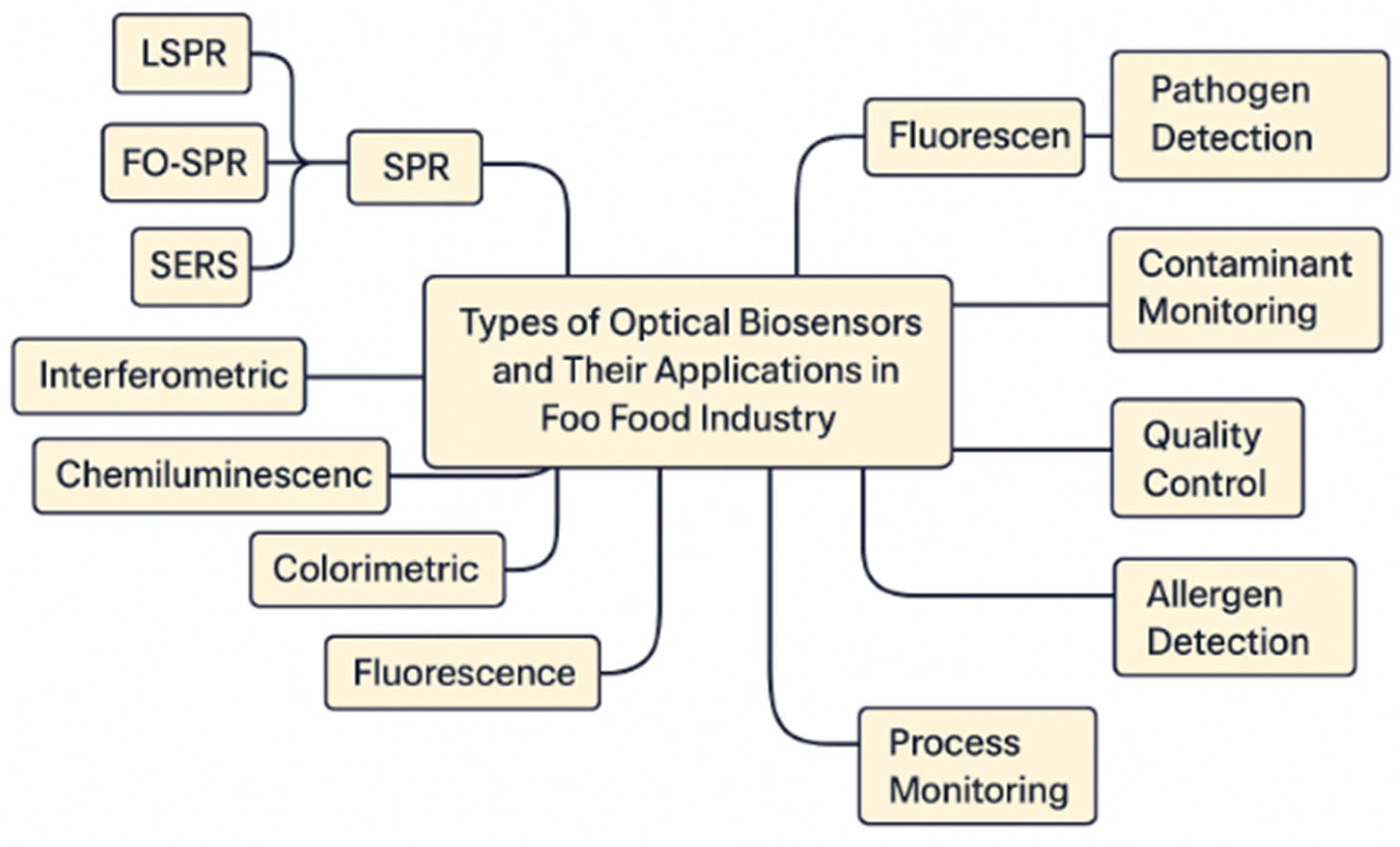
Optical biosensors (as shown in Figure 1) have become indispensable in the food industry due to their exceptional sensitivity, specificity, rapid response times, and capacity for real-time, non-invasive analysis. These biosensors function by converting a biological interaction into a measurable optical signal—such as fluorescence, absorbance, or refractive index shifts—enabling the precise detection of foodborne pathogens, contaminants, toxins, allergens, and quality indicators. The diversity in optical biosensor designs has allowed for broad applications across food safety, quality assurance, and process monitoring. One of the most extensively used types is the SPR biosensor, which detects changes in refractive index at the sensor surface as a result of molecular binding events. This type is widely utilized for detecting foodborne pathogens like E. coli, Salmonella, and Listeria, offering label-free and real-time detection [3,4]. A closely related variant, the Localized SPR biosensor, enhances detection sensitivity by incorporating metallic nanoparticles, which amplify local electromagnetic fields. LSPR sensors are particularly effective in detecting microbial contaminants and pesticide residues at trace levels [4]. Further refinement of SPR-based detection is achieved through Fiber Optic SPR (FO-SPR) biosensors, which use optical fibers to guide light and increase sensitivity in complex food matrices. These are especially suited for real-time quality assurance applications in liquid foods like milk or juices. Another highly sensitive technique is Surface-Enhanced Raman Scattering (SERS) biosensing, which uses metallic nanostructures to intensify the Raman scattering signal. SERS biosensors have been applied in the ultra-sensitive detection of microbial toxins and adulterants in food products [3]. Similarly, Surface-Enhanced Fluorescence (SEF) biosensors improve the fluorescence output of labeled targets, aiding in the detection of allergens, such as peanut proteins, and trace-level contaminants. Total Internal Reflection (TIR) biosensors, which rely on changes in light reflection at the interface of two media, offer excellent surface sensitivity and are used for rapid detection of bacterial contamination. Another category, Interferometric biosensors, utilizes the interference patterns of light to enable high-throughput and multiplex detection of several analytes simultaneously—ideal for screening multiple contaminants in food samples [11]. Fluorescence biosensors, based on detecting variations in fluorescent signals, have been used extensively for identifying toxins and mycotoxins, while chemiluminescence biosensors offer a simple yet effective platform for the detection of pathogens like Campylobacter or Listeria due to their low background noise and high signal contrast [3]. Colorimetric biosensors represent perhaps the most user-friendly class of optical biosensors, providing visible color changes in the presence of specific analytes, which allows even non-experts to interpret results easily. These are particularly effective in detecting spoilage indicators and adulterants in dairy and meat products [25,26]. The food industry applies these optical biosensors in a wide array of contexts. For pathogen detection, they serve as frontline tools in preventing outbreaks of foodborne illness, detecting microbial threats in raw materials and finished products [3,27]. For contaminant monitoring, biosensors are critical in screening for pesticide residues, veterinary drug residues, and toxic heavy metals, ensuring compliance with international food safety standards [23,27]. In quality control, biosensors can monitor biochemical changes such as sugar and alcohol levels during fermentation, or amino acid profiles in processed foods, which are important for flavor and shelf life [28]. Emerging applications include allergen detection, where biosensors are being deployed to trace minute quantities of allergens like gluten or peanut proteins in processed foods, thus improving food labeling accuracy and consumer safety [29]. In process monitoring, biosensors are embedded in food production lines to continuously monitor microbial growth or detect shifts in environmental conditions that may compromise safety. Recent trends in biosensor development include miniaturization, with the design of portable, handheld devices that enable rapid, on-site food testing in markets, warehouses, and farms [4]. Another growing direction is Internet of Things (IoT) integration, where smart biosensors connected to cloud systems enable continuous food traceability and remote surveillance of safety parameters across the supply chain [30]. Moreover, quantum biosensing is emerging as a cutting-edge approach that leverages quantum effects to enhance detection sensitivity and resolution, holding promise for the next generation of ultra-sensitive biosensors [15]. Optical biosensors represent a transformative technology in the food industry, enabling fast, accurate, and scalable solutions for safety assurance and quality control. As sensor technologies evolve, they will play an increasingly central role in ensuring the integrity, transparency, and sustainability of global food systems.
Figure 1.
Optical Biosensors Applications in food industry.
3. Optical Biosensors in Pathogen Detection for Food Safety
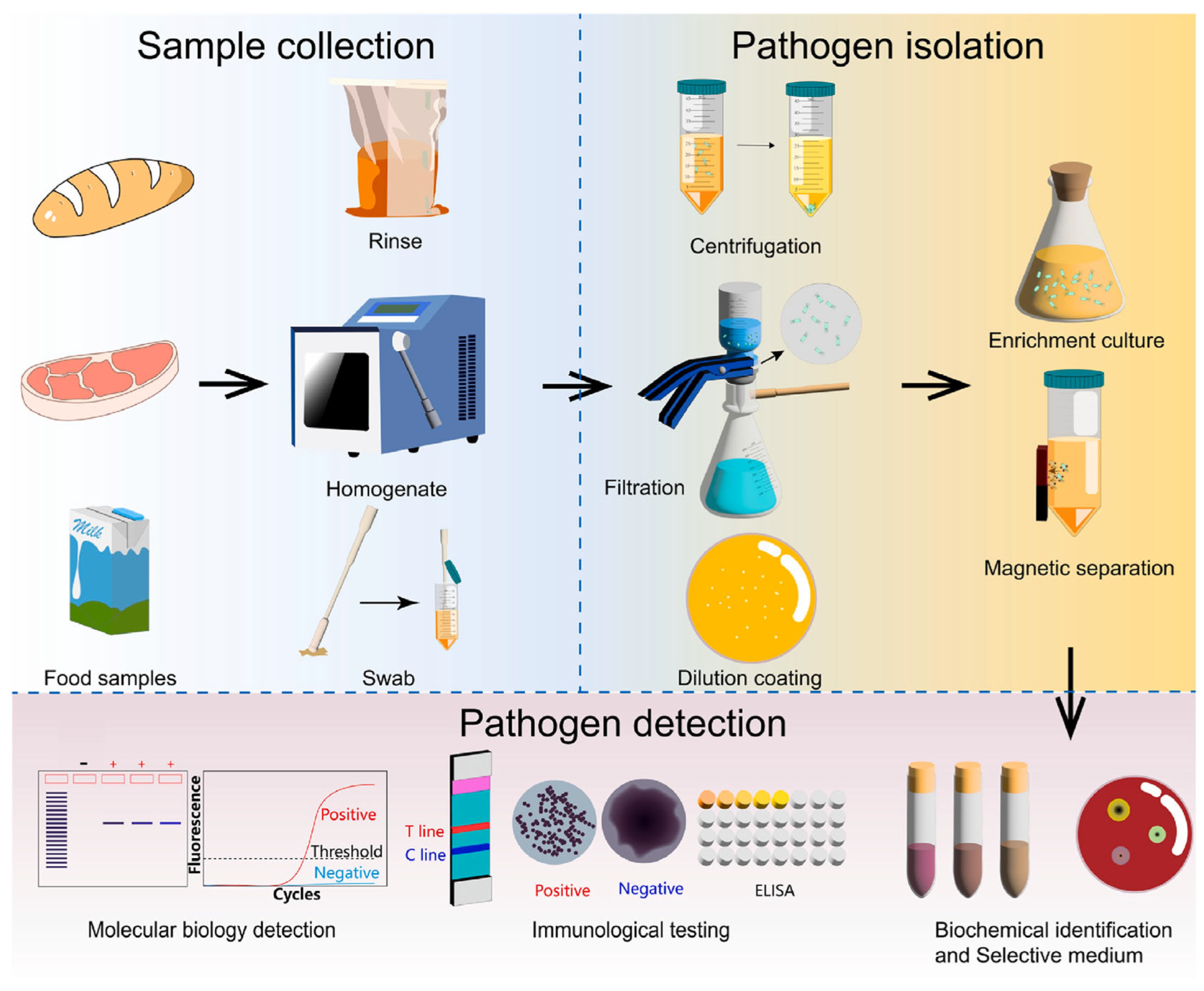
Optical biosensors are increasingly recognized as powerful tools for the detection of foodborne pathogens (as shown in Figure 2), offering rapid, sensitive, and cost-effective alternatives to traditional microbiological techniques. In the context of food safety, timely identification of pathogens such as Salmonella, Listeria monocytogenes, and Escherichia coli O157:H7 is critical for preventing disease outbreaks and ensuring compliance with food safety regulations. Optical biosensors function by converting a biological recognition event—such as the binding of a pathogen-specific antibody, aptamer, or nucleic acid—into an optical signal (e.g., fluorescence, color change, light emission, or refractive index shift), which can be easily measured and interpreted [3,31,32]. Several types of optical biosensors are employed for pathogen detection in food matrices, each offering distinct mechanisms of transduction and benefits. SPR biosensors detect minute changes in the refractive index at a metal–dielectric interface caused by the binding of pathogens, enabling label-free and real-time monitoring [3]. Fluorescence-based biosensors use fluorophore-labeled probes that emit light upon binding to target pathogens, achieving extremely high sensitivity and selectivity [33]. Surface-Enhanced Raman Scattering (SERS) biosensors, which amplify Raman signals using metallic nanostructures, enable the detection of pathogens at ultra-low concentrations, even in complex samples like milk or ground meat [33]. Colorimetric biosensors, on the other hand, provide simple visual readouts—typically a color change in the presence of the pathogen—making them especially useful for point-of-care or field-based applications [34]. Chemiluminescence biosensors, which generate light through a chemical reaction triggered by pathogen binding, offer high signal-to-noise ratios and fast analysis times [31]. Recent developments have focused on expanding the application of these biosensors across diverse food products, from raw produce to dairy and meat. The use of thin-film biosensor chips, disposable strips, and miniaturized readers has enabled faster on-site pathogen screening throughout the supply chain [32,34]. Integration with interferometric platforms, which use optical interference to detect binding events, has further enabled multiplexed detection of multiple pathogens in a single assay, increasing throughput and efficiency [11]. The incorporation of nanotechnology has significantly enhanced the capabilities of optical biosensors. Functionalized nanoparticles such as gold, silver, and magnetic nanomaterials serve as signal amplifiers and immobilization supports, offering improved detection limits and robustness in complex food matrices [31,33]. These nanostructures provide high surface-to-volume ratios, facilitating greater biorecognition probe density and faster interaction kinetics. For example, nanomaterials embedded in SERS or fluorescence platforms enable single-cell level detection of bacteria in food samples with minimal preprocessing [35]. Despite these advantages, challenges remain in fully optimizing optical biosensors for food safety monitoring. Issues such as matrix interference, cross-reactivity, and false positives or negatives can still affect analytical reliability, especially when detecting pathogens in processed or composite foods. Moreover, achieving uniform sensitivity across different sample types and scaling biosensor fabrication for commercial production are ongoing hurdles [31,36]. To address these, future research is focusing on the integration of advanced signal amplification techniques, such as enzyme-assisted reactions and CRISPR-based cascades, as well as the development of novel biorecognition elements like synthetic peptides and aptamer cocktails [36]. The comparative analysis of different optical biosensor types, including SPR, interferometric, fluorescence, and colorimetric approaches, is summarized in Table 1.
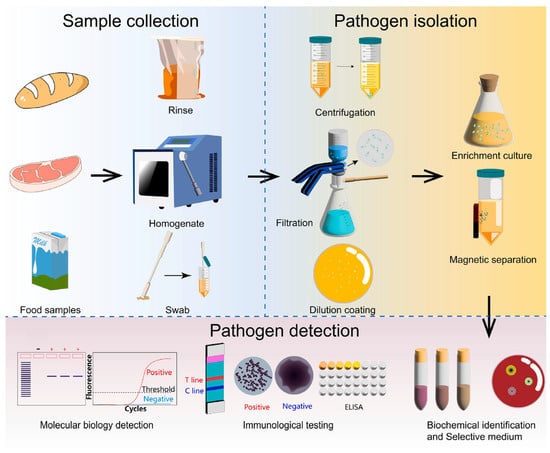
Figure 2.
The procedure of the detection of foodborne pathogens. Reprinted with permission from ref. [3]. Copyright 2024 Elsevier.

Table 1.
Comparative Performance Metrics and Suitability of Different Optical Biosensor Types.
Table 1 presents a comparative overview of the performance metrics and application suitability of four major classes of optical biosensors: Surface Plasmon Resonance (SPR), interferometric, fluorescence, and colorimetric systems. SPR biosensors are widely regarded for their label-free and real-time monitoring capabilities, achieving extremely low limits of detection (LOD) ranging between 0.05–10 mg/L depending on sensor design, surface chemistry, and chip materials [37,38,39]. These attributes make SPR highly versatile, with proven applicability in both laboratory and portable field formats [44]. However, the cost and system complexity remain moderate to high, especially when metal-coated chips or advanced calibration are required. Interferometric biosensors share the advantage of label-free detection but rely heavily on precise waveguide design and refractive index contrast for sensitivity. While they also support multiplexing and rapid detection, their complexity and dependence on sophisticated fabrication and alignment methods often restrict their applications to laboratory environments [40,41]. Fluorescence-based biosensors, on the other hand, are label-dependent and require the integration of fluorophores and detection optics, which adds cost and complexity but enables high sensitivity and multiplexing across proteins, nucleic acids, and viral biomarkers [33,42]. Their portability with handheld readers makes them particularly suitable for field diagnostics. Colorimetric biosensors provide a low-cost and user-friendly option, relying on visible changes in chromogenic reagents for detection. While they lack the ultra-sensitivity of SPR or fluorescence systems, their ease of use and naked-eye readouts make them practical for both laboratory and point-of-care applications, especially in resource-limited settings [33,42]. Their main drawback lies in limited multiplexing capacity, as only a restricted number of distinguishable color changes can be incorporated reliably.
4. Optical Biosensors in Toxin Detection for Food Safety
Optical biosensors have become increasingly valuable in the detection of foodborne toxins (as shown in Figure 3), offering a fast, sensitive, and portable alternative to conventional analytical techniques such as gas chromatography, liquid chromatography, and mass spectrometry. These biosensors leverage the interaction between light and specific biorecognition events—such as antibody–toxin or aptamer–toxin binding—to produce measurable optical signals. This enables the rapid identification of toxic compounds in food, which is essential for protecting public health, ensuring regulatory compliance, and minimizing the risk of foodborne illnesses [9,45]. Among the key advantages of optical biosensors is their exceptional sensitivity and specificity. Technologies based on chemiluminescence and fluorescence have demonstrated the ability to detect toxins such as aflatoxins in milk and cereal products at trace levels far below regulatory limits [9]. These detection methods benefit from signal amplification mechanisms that reduce interference from complex food matrices. Furthermore, optical biosensors enable real-time monitoring, making it possible to assess toxin presence during production, storage, and distribution [45,46]. This real-time capability allows for timely interventions that prevent contaminated products from reaching consumers. Another crucial benefit is portability—many optical biosensors are integrated into compact, user-friendly platforms that can be deployed for on-site testing, even in remote or resource-limited environments [11,47]. Different types of optical biosensors have been applied to toxin detection in food safety. Chemiluminescence and fluorescence biosensors are frequently used for aflatoxin detection due to their high signal-to-noise ratios and sensitivity [9]. Interferometric biosensors, which rely on light interference patterns, offer multiplex detection and are suitable for rapid screening of multiple toxins, including mycotoxins and bacterial toxins, in a single assay [11]. SPR biosensors, known for their label-free and real-time detection capabilities, are widely used to detect a broad range of contaminants such as microbial toxins, antibiotic residues, and pesticides in food products [48]. In addition, optical fiber biosensors offer high detection speed and flexibility by measuring the modulation of light signals as they interact with analytes in the sample. These are particularly suitable for detecting pollutants such as ochratoxins, fumonisins, and heavy metals in beverages and grains [8]. In terms of applications, optical biosensors have proven particularly useful in the detection of mycotoxins—toxic secondary metabolites produced by fungi like Aspergillus and Fusarium—which contaminate cereals, nuts, and dairy products. Mycotoxins such as aflatoxin B1 and ochratoxin A are highly carcinogenic and must be monitored closely to comply with safety standards [46,49]. Optical biosensors are also highly effective in detecting microbial toxins, such as staphylococcal enterotoxins, botulinum toxin, and toxins produced by Clostridium perfringens and E. coli O157:H7 [27,45]. Despite the promise of optical biosensors, several challenges must be addressed to facilitate their broader adoption. These include variability in sensor stability and reproducibility, potential false positives due to non-specific binding, and limitations in detecting ultra-low toxin concentrations in highly complex food matrices [50,51]. To overcome these limitations, current research is focused on the development of nanostructured materials, advanced signal amplification systems, and hybrid biorecognition elements like aptamers and molecularly imprinted polymers [36,45]. Future trends also point toward integrating optical biosensors with smartphone-based readout systems and IoT platforms, enabling real-time food surveillance and traceability in global food supply chains.
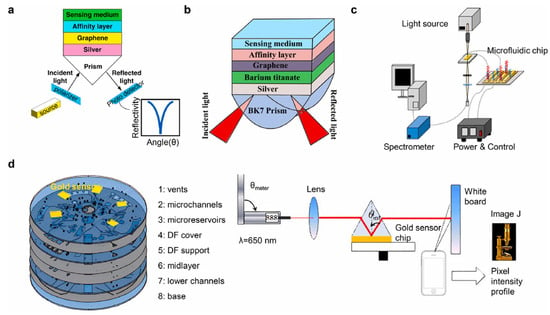
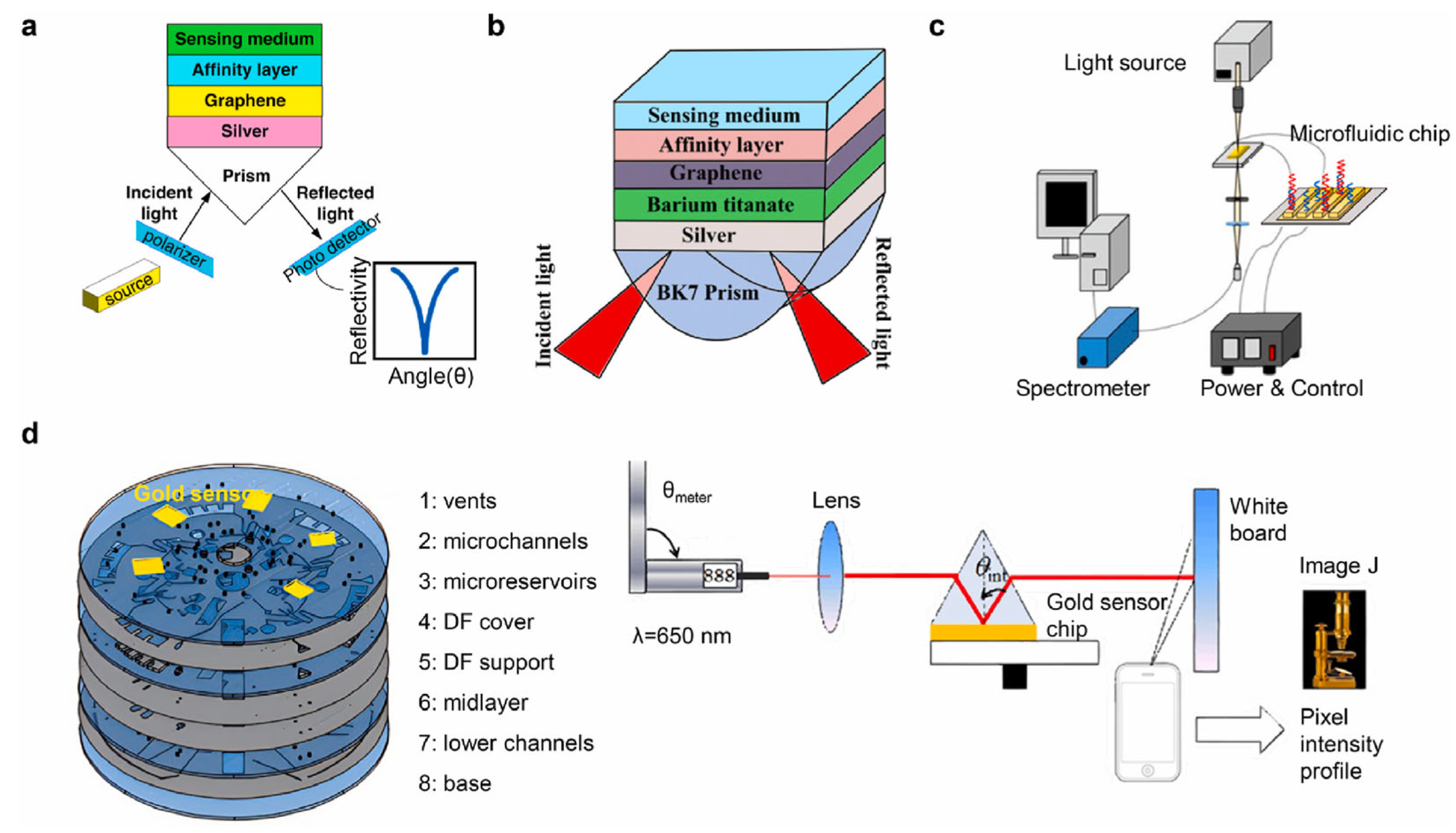
Figure 3.
SPR biosensors for the detection of foodborne pathogens. (a) Scheme of a prism/Ag/graphene/affinity layer/sensitive medium structure based SPR to detect E. coli. (b) Scheme of a prism/Ag/barium titanate/graphene/affinity layer/sensitive medium structure based SPR to detect P. aeruginosa (c) Scheme of a nano-SPR microfluidic biosensing chip combined with PCR (d) Scheme of a centrifugal lab-on-a-disk based SPR device with a smartphone. Reprinted with permission from ref. [3]. Copyright 2024 Elsevier.
5. Optical Biosensors in Monitoring Pesticide and Antibiotic Residues for Food Safety
Optical biosensors are increasingly recognized as crucial tools for ensuring food safety, particularly in the detection of pesticide and antibiotic residues in agricultural and animal-derived products. These biosensors, which operate by converting biochemical interactions into measurable optical signals, offer numerous advantages over traditional analytical methods such as chromatography and mass spectrometry. Their ability to provide rapid, real-time, and highly sensitive detection makes them ideal for modern food monitoring systems, especially in contexts requiring on-site analysis and timely interventions [2,52]. In the domain of pesticide residue detection, optical biosensors—particularly those based on SPR, optical fiber technologies, and fluorescent probes like carbon dots—have shown excellent performance in identifying harmful chemicals such as organophosphates and malathion across diverse food matrices, including milk, eggs, vegetables, and honey [8,53,54]. These biosensors use immobilized enzymes or antibodies to detect the presence of pesticide molecules with high specificity, and they generate detectable optical changes that correlate with residue concentrations. This ability to detect low-level contaminants in complex food environments is critical, particularly as pesticide misuse or overuse remains a global concern in agricultural practices.
Antibiotic residue detection is another area where optical biosensors are making substantial contributions. These sensors are capable of identifying veterinary drug residues such as aminoglycosides, tetracyclines, β-lactams, and β-agonists in meat, dairy, and egg products. SPR-based optical biosensors are widely used in this application due to their real-time monitoring capabilities and label-free detection mechanism [52,55,56]. Additionally, novel sensing platforms that incorporate carbon dots (CDs) and metal oxide nanozymes have demonstrated enhanced signal transduction and stability. Their unique optical properties, such as tunable fluorescence and photostability, allow for more precise quantification of trace-level antibiotic residues, addressing concerns related to antibiotic resistance and consumer health risks [55,57]. The advantages of optical biosensors in this context are manifold. Their high sensitivity and specificity make them suitable for detecting residues even at sub-regulatory thresholds, ensuring food products meet national and international safety standards [2,11]. Their portability and user-friendly formats—such as handheld readers and lateral flow devices—facilitate on-site testing, bypassing the need for expensive equipment and trained personnel. Furthermore, multiplexing capabilities, particularly in interferometric biosensor designs, allow simultaneous detection of multiple contaminants in a single assay, increasing efficiency and reducing testing time [11]. However, the implementation of optical biosensors for residue monitoring is not without challenges. One major limitation is matrix interference—the presence of fats, proteins, or other food components can hinder optical signal clarity and reduce assay accuracy [52]. To address this, ongoing research is exploring advanced surface chemistries and signal amplification strategies that enhance sensor robustness in complex sample environments. Another pressing challenge lies in sensor sensitivity and stability, especially when used for repeated or prolonged field applications. Innovations in nanomaterial engineering, such as the development of ultra-stable nanocomposites and biofunctional surfaces, are being actively pursued to overcome these issues [55,57]. One of the most promising future directions involves the integration of optical biosensors with machine learning (ML) and artificial intelligence (AI). These computational tools can analyze complex optical data, recognize patterns, and predict contamination levels with higher accuracy than conventional threshold-based methods. This smart integration can significantly improve the reliability and automation of food residue monitoring, particularly when scaled across supply chains or embedded into smart packaging systems [58].
6. Integration of Machine Learning and Artificial Intelligence in Optical Biosensor Technology for Food Safety
6.1. Integration of Artificial Intelligence
In recent years, the continuous accumulation of medical data has significantly expanded the application of artificial intelligence (AI) and machine learning (ML) in healthcare, elevating it to a broader and more practical level [59,60]. AI has emerged as a transformative force in food safety, particularly through its integration with optical biosensor technologies, enabling unprecedented improvements in contaminant detection, analytical precision, and real-time monitoring. By leveraging ML and deep learning (DL) algorithms, AI enhances biosensor performance through advanced pattern recognition, noise reduction, and signal optimization, thereby increasing both detection accuracy and sensitivity when identifying foodborne pathogens, toxins, pesticides, and antibiotic residues [61,62,63]. Optical biosensors such as surface plasmon resonance (SPR) sensors benefit significantly from AI-assisted signal processing, which refines sensor reliability, improves signal-to-noise ratios, and facilitates the interpretation of complex, multidimensional datasets [63,64]. Beyond laboratory analysis, AI-powered biosensors have enabled rapid, on-site detection capabilities, allowing for real-time food safety monitoring and immediate intervention to prevent contamination-related outbreaks [61,62,65]. Recent advancements such as smartphone-integrated nanozyme technologies (S-INTs) paired with AI have further improved portability and user accessibility, making high-sensitivity detection tools available in diverse food supply chain environments [66]. Moreover, AI has been increasingly integrated with complementary technologies including the Internet of Things (IoT), blockchain, and nanotechnology to establish intelligent, automated food safety ecosystems that deliver end-to-end traceability, predictive analytics, and enhanced governance of food safety protocols [61,67,68]. For instance, the combination of AI algorithms with photonic crystal fibers (PCFs) in SPR-based optical biosensors optimizes light propagation and increases detection accuracy, expanding the applicability of these platforms in quality assurance contexts [64]. Despite these advancements, the widespread adoption of AI-powered optical biosensors faces challenges, including scalability, cost-effectiveness, device compatibility, and regulatory compliance [61,65]. Ongoing research is focused on developing robust nanostructures, advanced surface coatings, and adaptive AI algorithms capable of seamlessly integrating with various biosensor architectures [64,69]. Additionally, the sustainability of these systems depends on coordinated efforts among stakeholders, regulatory bodies, and the public to ensure responsible deployment and long-term viability [61,67]. As AI-driven biosensing platforms continue to evolve, they hold the potential to revolutionize food safety monitoring by providing rapid, precise, and intelligent detection capabilities that protect public health and strengthen global food security.
6.2. Integration of Machine Learning in Optical Biosensor Technology for Food Safety
Machine learning (ML) has become a pivotal enabler in advancing optical biosensor technology for food safety, significantly enhancing sensitivity, specificity, and operational efficiency in detecting pathogens and contaminants across complex food matrices. By integrating ML algorithms with biosensing platforms, researchers have accelerated the detection of critical foodborne pathogens such as Escherichia coli and Staphylococcus aureus, enabling real-time monitoring and rapid response to contamination events while maintaining high analytical accuracy [70,71]. Optical biosensors, particularly those utilizing surface plasmon resonance (SPR) and surface-enhanced Raman scattering (SERS), benefit from ML’s advanced data processing capabilities, which enable accurate identification of low molecular weight contaminants even in challenging sample environments [63,72]. One of the key strengths of ML lies in its ability to process noisy or low-resolution biosensor signals, thereby enhancing reliability, improving signal-to-noise ratios, and facilitating more robust detection outcomes [63]. Deep learning models, in particular, have proven effective in extracting critical features from high-dimensional datasets generated by optical techniques such as near-infrared and hyperspectral imaging spectroscopy, enabling non-destructive and precise food quality assessment [73]. Coupling ML with the Internet of Things (IoT) has allowed for the creation of interconnected, intelligent food safety networks capable of continuous monitoring, automated quality control, and enhanced traceability throughout the supply chain [61,70]. These advancements extend into the development of AI-sensing nanosensors and smart packaging systems, which autonomously detect spoilage or contamination and provide real-time alerts, offering both regulatory compliance benefits and improved consumer safety [61]. Despite these innovations, challenges remain in ensuring high-quality, representative training datasets, improving the interpretability of ML models for regulatory acceptance, scaling up cost-effective production, and addressing data privacy and ethical concerns in connected monitoring systems [70,74]. Recent developments in multi-task Quantitative Structure–Toxicity Relationship (QSTR) models for acute toxicity prediction using machine learning (ML) demonstrate promising potential for integration with biosensor technologies. Such advancements could significantly enhance the predictive capabilities of biosensors, enabling more precise and reliable toxicity assessment in complex food matrices [75]. Continued research that bridges ML with cutting-edge innovations in nanotechnology, optical engineering, and automated food inspection systems will be essential to overcoming current barriers. This interdisciplinary approach will be critical for fully realizing the transformative potential of ML-powered optical biosensors in safeguarding global food supplies.
7. Challenges of Optical Biosensors in Food Safety Monitoring
Despite the considerable promise of optical biosensors in food safety applications, several critical challenges continue to impede their large-scale deployment, long-term stability, and regulatory acceptance. While these biosensors offer fast, sensitive, and real-time detection of contaminants such as pesticides, pathogens, toxins, and antibiotic residues, translating laboratory innovations into field-ready, industry-validated devices remains complex and multifaceted.
One of the primary challenges is achieving adequate sensitivity and specificity, especially when biosensors are used to detect trace levels of contaminants in complex food matrices. Food samples often contain proteins, fats, sugars, and other interfering substances that can mask target analytes or interact with sensor components, leading to signal suppression or false readings [7,13]. In such matrices, even state-of-the-art sensors can face difficulty distinguishing between the target molecule and background noise, thereby compromising detection accuracy. Moreover, specificity remains a bottleneck, as cross-reactivity with structurally similar compounds can result in false positives or false negatives, especially in multiplexed systems designed to detect multiple analytes simultaneously [11,31,76]. Another persistent issue is matrix interference, which varies widely depending on the food product. For instance, the complex composition of milk, meat, or processed foods can drastically affect biosensor performance. Ensuring consistent sensor responses across different types of food products remains a technical hurdle that must be addressed through improved sample preparation, advanced filtering strategies, or the development of highly selective recognition elements [7,12]. Stability and shelf life also present considerable limitations for real-world deployment. Many biosensors rely on biological recognition elements such as enzymes or antibodies that are inherently sensitive to temperature, humidity, and pH fluctuations. These biological components can degrade over time, particularly under non-ideal storage conditions, resulting in reduced sensor performance or complete failure [13,77]. This lack of stability restricts their usability in field settings or regions without cold-chain logistics. From a commercial standpoint, cost and scalability remain critical barriers. Although biosensors offer cost advantages over conventional laboratory techniques in the long run, their initial development, fabrication, and calibration can be expensive, especially when incorporating advanced nanomaterials or microfluidic platforms [77]. Moreover, scaling up production while maintaining quality control and sensor reproducibility is a significant challenge, particularly for applications requiring batch-to-batch consistency across thousands of units [78]. The regulatory landscape and lack of standardization further complicate the translation of optical biosensors into routine food safety protocols. Currently, there is no universal framework for validating or certifying biosensor-based methods across different food sectors, which creates hesitancy among manufacturers and food safety authorities. Regulatory approval processes are often lengthy and demanding, requiring extensive validation studies, inter-laboratory comparisons, and compliance with diverse regional standards [13,77,79]. To overcome these limitations, technological integration offers a promising path forward. Incorporating nanotechnology and artificial intelligence (AI) into biosensor platforms can enhance detection limits, automate data analysis, and reduce the incidence of false results [62]. Additionally, embedding biosensors into IoT-enabled smart packaging or automated food inspection systems may transform how real-time food safety monitoring is conducted on a global scale [31]. Nonetheless, to realize such visions, continued investment is needed in materials science, biosensor engineering, and computational analytics, as well as in creating global standards that can validate biosensor technologies across different food systems.
8. Current Status and Challenges of Standardizing Optical Biosensor Methods for Food Safety Control
Optical biosensors are increasingly recognized as promising analytical tools for food safety control, owing to their ability to combine high sensitivity, rapid detection, and real-time monitoring within compact platforms. These devices exploit light–biomolecule interactions to detect contaminants including pathogens, mycotoxins, heavy metals, antibiotic residues, and pesticides [6,7,8,11]. While the research base continues to expand, their regulatory acceptance and integration into official food control frameworks remain a major hurdle.
8.1. Current Status
Technological Progress.: Optical biosensors have evolved from laboratory prototypes to increasingly robust detection platforms. Surface plasmon resonance (SPR)-based sensors and other label-free detection strategies provide rapid, sensitive, and specific analysis of food contaminants [6,48]. Cutting-edge advances, such as nanotechnology integration, plasmonic enhancement, and microfluidics, have further improved miniaturization and portability [80].
Practical Applications.: Applications span across detection of foodborne pathogens, pesticide residues, toxins, and heavy metals [7,50,81]. The capability of optical biosensors to conduct real-time, on-site analysis aligns with global priorities for farm-to-fork safety and supply chain integrity [13]. Their potential to replace lengthy laboratory-based methods positions them as attractive candidates for official monitoring.
8.2. Challenges
Sensitivity and Matrix Effects.: Although optical biosensors offer remarkable sensitivity, their performance can be significantly impacted by food matrix interferences, leading to reduced specificity in complex samples [7,13]. Developing sensors that maintain reproducibility and robustness across diverse food types is a continuing challenge.
Regulatory Acceptance: Despite technological maturity, biosensors lack standardized validation protocols for official food control. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), the European Food Safety Authority (EFSA), and CODEX Alimentarius require rigorous method validation comparable to established techniques like HPLC and ELISA [82,83,84]. The absence of internationally harmonized guidelines delays regulatory adoption [85].
Cost and Commercialization.: Many optical biosensors remain at the prototype stage. High development and production costs, coupled with the need for user-friendly operation, hinder large-scale commercialization. Sustainable business models must balance market demand and technology performance to ensure real-world adoption [86,87].
8.3. Regulatory Considerations
FDA Perspective: The FDA follows a risk-based evaluation framework for new food testing methods, requiring demonstration of accuracy, reproducibility, and safety. The lengthy approval process contributes to delays in bringing biosensor technologies into regulatory practice [88]. EFSA and the EU: EFSA seeks harmonization of scientific evaluations but has historically faced delays and inconsistencies in applying novel technologies to food safety assessments [82,84]. The evaluation of biosensors often involves complex negotiations among stakeholders, slowing regulatory pathways. CODEX and Global Harmonization.: The CODEX Alimentarius Commission provides an international reference for food safety standards. However, biosensors are not yet fully embedded within CODEX methods. Recent policy discussions stress the need for globally harmonized protocols to facilitate international trade and acceptance of biosensor-based food monitoring [89].
9. Future Directions of Optical Biosensors in Food Safety and Potential Impact
9.1. Future Directions
The future of optical biosensors in food safety lies at the intersection of innovation in nanotechnology, microfabrication, digital integration, and multiplexed sensing. These advancements are reshaping how contaminants such as pathogens, toxins, pesticides, and antibiotic residues are detected in food, aiming to create rapid, sensitive, and field-deployable solutions for real-time monitoring across the entire food supply chain. One of the most transformative directions involves the integration of nanotechnology with optical biosensor platforms. Nanomaterials such as gold nanoparticles, quantum dots, graphene, carbon dots, and metal–organic frameworks (MOFs) are increasingly being embedded into optical sensing systems to enhance their sensitivity, specificity, and signal amplification [10,90]. These materials provide unique optical, electronic, and catalytic properties that allow for the detection of analytes at extremely low concentrations. Nanozymes, particularly metal oxide-based artificial enzymes, are also gaining attention due to their cost-effectiveness, environmental stability, and tunable functionalities, which support robust and repeatable sensing assays [57]. Another prominent trend is the miniaturization and development of portable biosensing devices. As food safety monitoring moves closer to the point of consumption and processing, biosensors are being designed in compact formats that integrate light sources, detectors, and microfluidic systems onto a single chip [5]. These lab-on-chip systems enable rapid, on-site testing and are particularly useful for perishable goods, where timely analysis is critical. Paired with battery-operated readers or smartphone-based interfaces, these devices have the potential to revolutionize food safety practices, especially in remote or resource-limited environments. The move toward multiplexed detection is another essential direction. Advanced optical biosensors are now being engineered to detect multiple analytes—such as a combination of pathogens, toxins, and allergens—simultaneously in a single assay [11,25]. Techniques like interferometric biosensing and SPR are being refined to support such multiplexing while maintaining high throughput and low cross-reactivity. This approach is particularly important in complex food matrices where various contaminants may co-occur. Parallel to these hardware advancements is the integration of biosensors with the IoT and smart monitoring systems. Real-time data acquisition, remote accessibility, and cloud-based analytics are enabling dynamic monitoring of food safety conditions throughout the supply chain—from farm to table [13]. This integration not only improves traceability but also facilitates predictive analytics and early-warning systems for contamination events. However, as the technology evolves, challenges such as stability, reproducibility, and regulatory compliance must be addressed. Ensuring that biosensors maintain performance over time and across different environmental conditions is critical for their widespread deployment. Moreover, regulatory frameworks must evolve to accommodate these emerging technologies, establishing standardized protocols for validation and performance benchmarking [6,48].
9.2. Potential Impact
The integration of nanotechnology, microfabrication, multiplexed sensing, and digital connectivity into optical biosensor platforms has the potential to revolutionize food safety monitoring. These innovations can deliver rapid, ultra-sensitive, and portable detection of diverse contaminants—such as pathogens, toxins, pesticides, and antibiotics—directly at critical points in the food supply chain. Nanomaterials, including gold nanoparticles, quantum dots, graphene, carbon dots, and MOFs, enhance detection limits and signal stability, while lab-on-chip systems enable compact, field-deployable devices for timely testing of perishable goods. Multiplexed detection allows simultaneous analysis of multiple contaminants, improving efficiency in complex food matrices. When coupled with IoT-enabled monitoring and cloud-based analytics, these biosensors support real-time traceability, predictive risk assessment, and early contamination warnings. Such advancements can strengthen consumer protection, reduce foodborne illness outbreaks, and enhance global food security, particularly in resource-limited and high-risk settings [77,82,85].
10. Conclusions
Optical biosensors have rapidly become essential in modern food safety and quality assurance, owing to their ability to convert biological interactions into measurable optical signals with high sensitivity, specificity, and speed. These devices enable precise detection of diverse contaminants, including pathogens, toxins, pesticides, and antibiotics. Techniques such as surface plasmon resonance (SPR), surface-enhanced Raman scattering (SERS), fluorescence, and interferometric sensing offer real-time, label-free, and multiplexed analysis across the entire food supply chain, from raw material inspection to post-processing quality control, thereby reducing the risk of foodborne illness and contamination. Advances in nanotechnology have significantly enhanced sensor performance, with quantum dots, carbon dots, and metal–organic frameworks improving detection sensitivity, stability, and miniaturization. Coupled with innovations in microfluidics, machine learning, and IoT integration, optical biosensors are evolving into intelligent, connected platforms capable of continuous monitoring and predictive analytics. Despite these advances, challenges remain in reproducibility, scalability, and regulatory validation, alongside technical barriers such as matrix interference, sensor degradation, and the absence of standardized protocols. Interdisciplinary research combining material science, biotechnology, and digital analytics is actively addressing these limitations. The future lies in seamlessly integrating optical biosensors into automated, AI-driven monitoring systems, delivering precise, real-time safety insights for proactive food protection.
Author Contributions
Conceptualization, P.R.B. and P.P.B.; methodology, P.R.B.; software, P.R.B.; validation, P.R.B., P.P.B. and E.B.; formal analysis, P.R.B.; investigation, P.R.B.; resources, E.B.; data curation, P.R.B.; writing—original draft preparation, P.R.B.; writing—review and editing, P.P.B. and E.B.; visualization, P.R.B.; supervision, P.P.B.; project administration, P.P.B.; funding acquisition, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anish Kumar, M.; Jung, S.; Ji, T. Protein biosensors based on polymer nanowires, carbon nanotubes and zinc oxide nanorods. Sensors 2011, 11, 5087–5111. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.P.; Maity, S. Optical biosensors: Principles, techniques, sensor design and their application in food analysis. In Biosensors in Food Safety and Quality: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2022; pp. 23–36. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, K.; Lin, J. Optical biosensors for the detection of foodborne pathogens: Recent development and future prospects. TrAC Trends Anal. Chem. 2024, 177, 117785. [Google Scholar] [CrossRef]
- Balbinot, S.; Srivastav, A.M.; Vidic, J.; Abdulhalim, I.; Manzano, M. Plasmonic biosensors for food control. Trends Food Sci. Technol. 2021, 111, 128–140. [Google Scholar] [CrossRef]
- Raptis, I.; Misiakos, K.; Makarona, E.; Salapatas, A.; Petrou, P.; Kakabakos, S.; Botsialas, A.; Jobst, G.; Haasnoot, W.; Fernandez-Alba, A.; et al. A miniaturized optoelectronic system for rapid quantitative label-free detection of harmful species in food. In Proceedings of the Progress in Biomedical Optics and Imaging—Proceedings of SPIE, San Francisco, CA, USA, 13–14 February 2016; Volume 9725, p. 97250A. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-free optical biosensors for food and biological sensor applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance—A review. J. Food Sci. Technol. 2012, 49, 383–406. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Y.; Tang, S.; Kong, D.; Liu, C. Construction and application in food contaminants detection of novel optical fiber biosensors. Prog. Chem. 2024, 36, 120–131. [Google Scholar] [CrossRef]
- Bhand, S.; Kanungo, L.; Pal, S. Chapter 7: Chemiluminescence and fluorescence optical biosensor for the detection of aflatoxins in food. In Food Chemistry, Function and Analysis; Royal Society of Chemistry: London, UK, 2017; Volume 2017, pp. 161–181. [Google Scholar] [CrossRef]
- Zheng, L.; Jin, W.; Xiong, K.; Zhen, H.; Li, M.; Hu, Y. Nanomaterial-based biosensors for the detection of foodborne bacteria: A review. Analyst 2023, 148, 5790–5804. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Petrou, P.; Kakabakos, S. Advances in interferometric sensors for the detection of food contaminants. TrAC Trends Anal. Chem. 2024, 175, 117714. [Google Scholar] [CrossRef]
- Moran, K.L.M.; Fitzgerald, J.; McPartlin, D.A.; Loftus, J.H.; O’Kennedy, R. Biosensor-based technologies for the detection of pathogens and toxins. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 74, pp. 93–120. [Google Scholar] [CrossRef]
- Nath, S. Advancements in food quality monitoring: Integrating biosensors for precision detection. Sustain. Food Technol. 2024, 2, 976–992. [Google Scholar] [CrossRef]
- Caratelli, V.; Di Meo, E.; Colozza, N.; Fabiani, L.; Fiore, L.; Moscone, D.; Arduini, F. Nanomaterials and paper-based electrochemical devices: Merging strategies for fostering sustainable detection of biomarkers. J. Mater. Chem. B 2022, 10, 9021–9039. [Google Scholar] [CrossRef]
- Sagar Shrikrishna, N.; Sharma, R.; Sahoo, J.; Kaushik, A.; Gandhi, S. Navigating the landscape of optical biosensors. Chem. Eng. J. 2024, 490, 151661. [Google Scholar] [CrossRef]
- Lugongolo, M.Y.; Ombinda-Lemboumba, S.; Mthunzi-Kufa, P. Optical biosensing of human immunodeficiency virus on a gold coated surface. In Proceedings of the Progress in Biomedical Optics and Imaging—Proceedings of SPIE, San Francisco, CA, USA, 28 January–2 February 2023; Volume 12396, p. 123960B. [Google Scholar] [CrossRef]
- Maphanga, C.; Manoto, S.; Ombinda-Lemboumba, S.; Hlekelele, L.; Mthunzi-Kufa, P. Optical biosensing of mycobacterium tuberculosis for point-of-care diagnosis. In Proceedings of the Progress in Biomedical Optics and Imaging—Proceedings of SPIE, San Francisco, CA, USA, 1–6 February 2020; Volume 11251, p. 112510R. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, X.; Zhang, Y.; Gu, C.; Song, B.; Shi, H. Kinetic analysis of a high-affinity antibody/antigen interaction performed by planar waveguide fluorescence immunosensor. RSC Adv. 2016, 6, 13837–13845. [Google Scholar] [CrossRef]
- Palchetti, I.; Mascini, M. Biosensor technology: A brief history. In Sensors and Microsystems; Lecture Notes in Electrical Engineering; Springer: Dordrecht, The Netherlands, 2010; Volume 54, pp. 15–23. [Google Scholar] [CrossRef]
- De Marcos, S.; Sanz, V.; Andreu, Y.; Galbán, J. Comparative study of polymeric supports as the base of immobilisation of chemically modified enzymes. Microchim. Acta 2006, 153, 163–170. [Google Scholar] [CrossRef]
- Bertucci, C.; Piccoli, A.; Pistolozzi, M. Optical biosensors as a tool for early determination of absorption and distribution parameters of lead candidates and drugs. Comb. Chem. High Throughput Screen. 2007, 10, 433–440. [Google Scholar] [CrossRef]
- Bosch, M.E.; Sánchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Optical chemical biosensors for high throughput screening of drugs. Comb. Chem. High Throughput Screen. 2007, 10, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kumar, S.; Nedoma, J.; Martinek, R.; Marques, C. Advancements in optical biosensing techniques: From fundamentals to future prospects. APL Photonics 2024, 9, 091102. [Google Scholar] [CrossRef]
- Zamarreño, C.R.; Socorro, A.B.; Sanchez, P.; Matias, I.R.; Arregui, F.J. Optical fibers: Biosensors. In Encyclopedia of Optical and Photonic Engineering, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 2034–2052. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Ying, Y.; Li, Y. Recent advances in biosensors for food safety detection. NongyeGongchengXuebao/Trans. Chin. Soc. Agric. Eng. 2007, 23, 272–277. [Google Scholar]
- Singh, P.; Pandey, V.K.; Srivastava, S.; Singh, R. A systematic review on recent trends and perspectives of biosensors in food industries. J. Food Saf. 2023, 43, e13071. [Google Scholar] [CrossRef]
- Rasooly, A.; Herold, K.E. Biosensors for the analysis of food- and waterborne pathogens and their toxins. J. AOAC Int. 2006, 89, 873–883. [Google Scholar] [CrossRef]
- Terry, L.A.; White, S.F.; Tigwell, L.J. The application of biosensors to fresh produce and the wider food industry. J. Agric. Food Chem. 2005, 53, 1309–1316. [Google Scholar] [CrossRef]
- Varzakas, T.; Nikoleli, G.-P.; Tzamtzis, N.; Nikolelis, D.P. Optical biosensors in food safety and control. In Portable Biosensing of Food Toxicants and Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2013; pp. 473–485. [Google Scholar] [CrossRef]
- Sehgal, S.; Aggarwal, S.; Saini, A.; Thakur, M.; Soni, K. Smart monitoring and surveillance of food contamination. In Smart and Sustainable Food Technologies; Springer: Singapore, 2022; pp. 263–285. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Zhu, Y.; Wan, J.; Wang, X.; Zhou, X.; Li, X.; Zhou, W. Research progress on multiplexed pathogen detection using optical biosensors. Biosensors 2025, 15, 378. [Google Scholar] [CrossRef]
- Choudari, S.; Subha, L.; Rashmi, N.; Chandrakar, M.K.; Pradeeshkumar, T.; Singh, A.; Yadav, H.S. Optical biosensors for rapid detection of foodborne pathogens in agricultural products. J. Appl. Bioanal. 2024, 10, 33–38. [Google Scholar]
- Oushyani Roudsari, Z.; Karami, Y.; Khoramrooz, S.S.; Rouhi, S.; Ghasem, H.; Khatami, S.H.; Alizadeh, M.; Ahmad Khosravi, N.; Mansoriyan, A.; Ghasemi, E.; et al. Electrochemical and optical biosensors for the detection of E. coli. Clin. Chim. Acta 2025, 565, 119984. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhao, J.; Zhang, Y.; Huang, W.; Xu, S.; Chen, H.; Fan, L.-M.; Chen, Y.; Deng, X.W. Rapid and reliable detection of 11 food-borne pathogens using thin-film biosensor chips. Appl. Microbiol. Biotechnol. 2010, 86, 983–990. [Google Scholar] [CrossRef]
- Habimana, J.D.D.; Ji, J.; Sun, X. Minireview: Trends in optical-based biosensors for point-of-care bacterial pathogen detection for food safety and clinical diagnostics. Anal. Lett. 2018, 51, 2933–2966. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Y.; Lin, J.; Liu, Y. Biosensors coupled with signal amplification technology for the detection of pathogenic bacteria: A review. Biosensors 2021, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Sekhwama, M.; Mpofu, K.; Sivarasu, S.; Mthunzi-Kufa, P. Enhancing limit of detection in surface plasmon resonance biosensors: A sensitivity analysis for optimal performance. In Proceedings of the Progress in Biomedical Optics and Imaging—Proceedings of SPIE, San Francisco, CA, USA, 27–28 January 2024; Volume 12840, p. 128400C. [Google Scholar] [CrossRef]
- Uniyal, A.; Gotam, S.; Ram, T.; Chauhan, B.; Jha, A.; Pal, A. Next generation ultra-sensitive surface plasmon resonance biosensors. In Communications in Computer and Information Science, Proceedings of the International Conference on Machine Learning, Image Processing, Network Security and Data Sciences, Bhopal, India, 21–22 December 2022; Springer: Cham, Switzerland, 2023; Volume 1762, pp. 353–361. [Google Scholar] [CrossRef]
- Granqvist, N.; Hanning, A.; Eng, L.; Tuppurainen, J.; Viitala, T. Label-enhanced surface plasmon resonance: A new concept for improved performance in optical biosensor analysis. Sensors 2013, 13, 15348–15363. [Google Scholar] [CrossRef]
- Daghestani, H.N.; Day, B.W. Theory and applications of surface plasmon resonance, resonant mirror, resonant waveguide grating, and dual polarization interferometry biosensors. Sensors 2010, 10, 9630–9646. [Google Scholar] [CrossRef]
- Calò, G.; Farinola, A.; Petruzzelli, V. Design and optimization of high sensitivity photonic interferometric biosensors on polymeric waveguides. Prog. Electromagn. Res. Lett. 2012, 33, 151–166. [Google Scholar] [CrossRef]
- Eksin, E.; Erdem, A. Recent progress on optical biosensors developed for nucleic acid detection related to infectious viral diseases. Micromachines 2023, 14, 295. [Google Scholar] [CrossRef]
- Lyshyk, N.F.; Tarodub, N.F. Comparison of the efficiency control of mycotoxins by some optical immune biosensors. In Proceedings of the Progress in Biomedical Optics and Imaging—Proceedings of SPIE, Riga, Latvia, 26–31 August 2013; Volume 9032, p. 903210. [Google Scholar] [CrossRef]
- Moreira, C.S.; Oliveira, L.C.; Fischer, R.; Medeiros, E.S.; Lima, A.M.N.; Neff, H. Polymer-based surface plasmon resonance biochip: Construction and experimental aspects. Res. Biomed. Eng. 2016, 32, 92–103. [Google Scholar] [CrossRef]
- Yi, Z.; Ren, Y.; Li, Y.; Li, Y.; Long, F.; Zhu, A. Optical biosensors for microbial toxin detection: Recent advances and future trends. Microchem. J. 2023, 191, 108894. [Google Scholar] [CrossRef]
- Urs, D.; Madesh, A.; Mahmood, K.; Sreeharsha, N.; Dharmappa, K.K. Real-time utilization of nanostructured biosensors for the determination of food toxins. In Novel Nanostructured Materials for Electrochemical Bio-Sensing Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 367–378. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Lee, C.-C.; Pourjafar, H.; Ansari, F.; Alizadeh Sani, M.; Ajili, N.; Assadpour, E.; Zhang, F.; Jafari, S.M. Nano-immunosensors for the rapid and sensitive detection of foodborne toxins; Recent advances. Ind. Crops Prod. 2025, 228, 120879. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar, S.; M, Y.; S, R.; C A, M.; Thirunavookarasu, S.N.; C K, S. Recent advances in Surface Plasmon Resonance (SPR) biosensors for food analysis: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1055–1077. [Google Scholar] [CrossRef]
- Tang, X.; Zuo, J.; Yang, C.; Jiang, J.; Zhang, Q.; Ping, J.; Li, P. Current trends in biosensors for biotoxins (mycotoxins, marine toxins, and bacterial food toxins): Principles, application, and perspective. TrAC Trends Anal. Chem. 2023, 165, 117144. [Google Scholar] [CrossRef]
- Sharma, H.; Mutharasan, R. Review of biosensors for foodborne pathogens and toxins. Sens. Actuators B Chem. 2013, 183, 535–549. [Google Scholar] [CrossRef]
- Yang, M.; Sapsford, K.E.; Sergeev, N.; Sun, S.; Rasooly, A. Meeting current public health needs: Optical biosensors for pathogen detection and analysis. In Proceedings of the Progress in Biomedical Optics and Imaging—Proceedings of SPIE, San Jose, CA, USA, 24–29 January 2009; Volume 7167, p. 716702. [Google Scholar] [CrossRef]
- Petz, M. Recent applications of surface plasmon resonance biosensors for analyzing residues and contaminants in food. Monatshefte Chem. 2009, 140, 953–964. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G. Advances in biosensor-based instruments for pesticide residues rapid detection. Int. J. Electrochem. Sci. 2015, 10, 9790–9807. [Google Scholar] [CrossRef]
- Utegenova, A.; Kakimova, Z.; Klivenko, A.; Kapshakbayeva, Z.; Imankulova, G.; Naurzbaeva, G.; Tulkebayeva, G.; Mirasheva, G. Acetylcholinesterase immobilized on glass rod for organophosphorus pesticides detection: Application on milk analysis. Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 843–848. [Google Scholar] [CrossRef]
- Zheng, J.; Lian, Z.; Liu, T.; Ouyang, M.; Jiang, S.; Yuan, X.; Zhou, L. A review for carbon dots-based fluorescent sensing tools for antibiotic and pesticide residues: Progress, challenge and perspective. Food Control 2025, 172, 111201. [Google Scholar] [CrossRef]
- Ma, J.; Lu, B.; Zhang, P.; Li, D.; Xu, K. Liquid transfer of graphene to the cylindrical gold nanostructures for sensitivity enhancements of SPR glucose sensor. Sens. Actuators A Phys. 2023, 353, 114227. [Google Scholar] [CrossRef]
- Qin, J.; Guo, N.; Yang, J.; Wei, J. Recent advances in metal oxide nanozyme-based optical biosensors for food safety assays. Food Chem. 2024, 447, 139019. [Google Scholar] [CrossRef]
- Hassan, M.M.; Xu, Y.; Sayada, J.; Zareef, M.; Shoaib, M.; Chen, X.; Li, H.; Chen, Q. Progress of machine learning-based biosensors for the monitoring of food safety: A review. Biosens. Bioelectron. 2025, 267, 116782. [Google Scholar] [CrossRef]
- Lin, H. Artificial intelligence with great potential in medical informatics: A brief review. Medinformatics 2024, 1, 2–9. [Google Scholar] [CrossRef]
- Chowdhury, S.H.; Mamun, M.; Shaikat, M.T.A.; Hussain, M.I.; Iqbal, M.S.; Hossain, M.M. An ensemble approach for artificial neural network-based liver disease identification from optimal features through hybrid modeling integrated with advanced explainable AI. Medinformatics 2025, 2, 107–119. [Google Scholar] [CrossRef]
- Pandhi, S.; Kumari, N.; Jain, A.; Sharma, V. Emerging technologies in food safety: AI-powered, nano-enabled, and biosensor-based strategies for rapid contaminant detection. Food Anal. Methods 2025, 18, 2010–2024. [Google Scholar] [CrossRef]
- Deng, Z.; Yun, Y.-H.; Duan, N.; Wu, S. Artificial intelligence algorithms-assisted biosensors in the detection of foodborne pathogenic bacteria: Recent advances and future trends. Trends Food Sci. Technol. 2025, 161, 105072. [Google Scholar] [CrossRef]
- Mohseni-Dargah, M.; Falahati, Z.; Dabirmanesh, B.; Nasrollahi, P.; Khajeh, K. Machine learning in surface plasmon resonance for environmental monitoring. In Artificial Intelligence and Data Science in Environmental Sensing; Elsevier: Amsterdam, The Netherlands, 2022; pp. 269–298. [Google Scholar] [CrossRef]
- Singh, H.P.; Singh, J.; Mukherjee, I.; Singh, S. Surface plasmon resonance-based optical biosensors for refractometric sensing: A theoretical review. In Proceedings of the 3rd IEEE International Conference on Device Intelligence, Computing and Communication Technologies (DICCT 2025), Dehradun, India, 21–22 March 2025; pp. 568–573. [Google Scholar] [CrossRef]
- Choudhary, R.; Rathore, N.; Parihar, K.; Chauhan, M.S.; Binani, S.; Kumar, N. Unveiling the nano world: Expanding food safety monitoring through nano-biosensor technology. J. Food Chem. Nanotechnol. 2024, 10, S94–S100. [Google Scholar] [CrossRef]
- Gbonyea, F.P.; Wu, J.; Li, M.; Liang, M.; Zhang, M.; Zhu, X.; Li, X.; He, S.; Liu, P. Smartphone-integrated nanozyme approaches for rapid and on-site detection: Empowering smart food safety. Food Chem. 2025, 486, 144678. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Tan, G.; Muhammad, R.; Liu, J.; Bi, J. AI-powered innovations in food safety from farm to fork. Foods 2025, 14, 1973. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Song, G.; Huang, K.; Luo, Y.; Liu, Q.; He, X.; Cheng, N. Intelligent biosensing strategies for rapid detection in food safety: A review. Biosens. Bioelectron. 2022, 202, 114003. [Google Scholar] [CrossRef]
- Sariçam, M. Crafting tomorrow’s diagnostics: Metal nanoparticles in biosensors—Types, synthesis, and future frontiers in health, environment, and food safety. In Handbook of Biosensors Technology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 69–109. [Google Scholar]
- Onyeaka, H.; Akinsemolu, A.; Miri, T.; Nnaji, N.D.; Emeka, C.; Tamasiga, P.; Pang, G.; Al-sharify, Z. Advancing food security: The role of machine learning in pathogen detection. Appl. Food Res. 2024, 4, 100532. [Google Scholar] [CrossRef]
- Xu, Y.; Ahmad, W.; Chen, M.; Wang, J.; Jiao, T.; Wei, J.; Chen, Q.; Li, D.; Chen, X.; Chen, Q. Active capture-directed bimetallic nanosubstrate for enhanced SERS detection of Staphylococcus aureus by combining strand exchange amplification and wavelength-selective machine learning. Biosens. Bioelectron. 2025, 278, 117363. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, J.; Jin, J.; Zhou, H.; Jin, S.; Yang, D. Advances in machine learning-assisted SERS sensing towards food safety and biomedical analysis. TrAC Trends Anal. Chem. 2024, 180, 117974. [Google Scholar] [CrossRef]
- Lee, I.-H.; Ma, L. Integrating machine learning, optical sensors, and robotics for advanced food quality assessment and food processing. Food Innov. Adv. 2025, 4, 65–72. [Google Scholar] [CrossRef]
- He, Q.; Huang, H.; Wang, Y. Detection technologies, and machine learning in food: Recent advances and future trends. Food Biosci. 2024, 62, 105558. [Google Scholar] [CrossRef]
- Kovalishyn, V.; Hodyna, D.; Metelytsia, L. Development of Multi-task QSTR Models for Acute Toxicity Prediction Towards Daphnia magna Using Machine Learning in the OCHEM Platform. Medinformatics 2025, 2, 93–98. [Google Scholar] [CrossRef]
- Luo, Y.; Nartker, S.; Miller, H.; Hochhalter, D.; Wiederoder, M.; Wiederoder, S.; Setterington, E.; Drzal, L.T.; Alocilja, E.C. Surface functionalization of electrospun nanofibers for detecting E. coli O157:H7 and BVDV cells in a direct-charge transfer biosensor. Biosens. Bioelectron. 2010, 26, 1612–1617. [Google Scholar] [CrossRef]
- Amin, N.; Almasi, A.; Ozer, T.; Henry, C.S.; Hosseinzadeh, L.; Keshavarzi, Z. Recent advances of optical biosensors in veterinary medicine: Moving towards the point of care applications. Curr. Top. Med. Chem. 2023, 23, 2242–2265. [Google Scholar] [CrossRef]
- Nastasijevic, I.; Kundacina, I.; Jaric, S.; Pavlovic, Z.; Radovic, M.; Radonic, V. Recent advances in biosensor technologies for meat production chain. Foods 2025, 14, 744. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Asiri, A.M.; Du, D.; Wen, W.; Wang, S.; Lin, Y. Nanomaterial-enhanced paper-based biosensors. TrAC Trends Anal. Chem. 2014, 58, 31–39. [Google Scholar] [CrossRef]
- Bari, A.; Aslam, S.; Khan, H.U.; Shakil, S.; Yaseen, M.; Shahid, S.; Yusaf, A.; Afshan, N.; Shafqat, S.S.; Zafar, M.N. Next-generation optical biosensors: Cutting-edge advances in optical detection methods. Plasmonics 2025. [Google Scholar] [CrossRef]
- Surbhi, G.; Sanjay, S.; Neeti, K. Aptamer-based optical sensors for food safety. In Surface Engineering and Functional Nanomaterials for Point-of-Care Analytical Devices; Elsevier: Amsterdam, The Netherlands, 2024; pp. 125–146. [Google Scholar] [CrossRef]
- Garcia-Vello, P.; Aiello, K.; Smith, N.M.; Fabrega, J.; Paraskevopoulos, K.; Hugas, M.; Heppner, C. Preparing for future challenges in risk assessment in the European Union. Trends Biotechnol. 2022, 40, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Levidow, L.; Carr, S. Europeanising advisory expertise: The role of “independent, objective, and transparent” scientific advice in agri-biotech regulation. Environ. Plan. C Gov. Policy 2007, 25, 880–895. [Google Scholar] [CrossRef]
- Vero, V.; Gasbarrini, A. The EFSA health claims “learning experience”. Int. J. Food Sci. Nutr. 2012, 63 (Suppl. 1), 14–16. [Google Scholar] [CrossRef]
- von Wright, A. Safety assessment of probiotics in the European Union (EU). In Lactic Acid Bacteria: Microbiological and Functional Aspects; CRC Press: Boca Raton, FL, USA, 2019; pp. 723–734. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Male, K.B.; Glennon, J.D. Biosensor technology: Technology push versus market pull. Biotechnol. Adv. 2008, 26, 492–500. [Google Scholar] [CrossRef]
- Caiazza, R.; Bigliardi, B. Business models for biosensors in the food industry. In Handbook of Cell Biosensors; Springer: Berlin/Heidelberg, Germany, 2021; pp. 659–678. [Google Scholar] [CrossRef]
- Hogan, J.M.; Kasser, M.; Prem, S. 7.42 Regulatory affairs. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 813–828. [Google Scholar] [CrossRef]
- Pawnikar, V.; Patel, M. Biosensors in wearable medical devices: Regulatory framework and compliance across US, EU, and Indian markets [Biocapteurs dans les dispositifs médicaux portables: Cadre réglementaire et conformité sur les marchés américain, européen et indien]. Ann. Pharm. Françaises 2025, 83, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.H.; Doan, M.Q.; Dinh, N.X.; Huy, T.Q.; Tri, D.Q.; Ngoc Loan, L.T.; Van Hao, B.; Le, A.-T. Gold nanoparticle-based optical nanosensors for food and health safety monitoring: Recent advances and future perspectives. RSC Adv. 2022, 12, 10950–10988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).