Abstract
Electrochemical aptasensors have been successfully applied in a number of fields, including food safety, environmental monitoring, and the health sector. They offer a robust and environmentally friendly alternative to antibody-based detection, with the added benefits of flexible design, high chemical and thermal stability, and low immunogenicity. In this work, we present an electrochemical aptasensor based on a semiconducting mesoporous TiO2:Mn working electrode (WE) for the sensitive detection of tetracycline (TET). The TiO2:Mn electrodes were fabricated using a scalable screen-printing process, providing a cost-efficient and reproducible platform for sensor development. Specifically, a 5 μM solution of the DNA aptamer with the sequence 5′-CCC CCG GCA GGC CAC GGC TTG GGTTGG TCC CAC TGC GCG-3′ was utilized for the detection of tetracycline (TET) in spiked aqueous samples, across a concentration range of 0.3 to 25.0 ng/mL. Detection was performed via differential pulse voltammetry (DPV) using a Pt wire cathode. The buffer used in the experiment was Tris–HCl (20 mM, pH 7.6), 100 mM of NaCl, MgCl2 (2 mM), KCl (5 mM), and CaCl2 (1 mM). The limit of detection (LOD) was calculated to be approximately 1 ng/mL.
1. Introduction
Electrochemical aptasensors have emerged as a highly versatile class of biosensors, offering sensitive, rapid, and cost-effective detection across diverse fields such as food safety, environmental surveillance, and healthcare [1,2]. These devices harness aptamers, i.e., synthetic ssDNA or RNA oligonucleotides, as biorecognition elements in place of traditional antibodies. Aptamers exhibit distinct advantages such as high specificity and affinity for their targets, chemical and thermal stability, as well as low immunogenicity. Moreover, their in vitro synthesis enables reproducible batch manufacturing with high fidelity, free from the biological variability often associated with antibodies. This synthetic flexibility allows for precise sequence design, easy chemical modification, and scalable production, thereby ensuring consistent performance across different biosensing platforms.
In particular, aptasensors have been widely investigated for the sensitive and selective detection of tetracycline (TET), demonstrating excellent analytical performance across various electrochemical platforms. Recent advances in electrochemical aptasensing have significantly enhanced the detection of tetracyclines in complex matrices such as milk and environmental samples. Raykova et al. [3] highlighted the potential of emerging electrochemical platforms for the real-time monitoring of tetracyclines in dairy products, underscoring the importance of rapid and portable detection systems. Building on this, Weng et al. [4] developed a high-performance sensor based on a Zr-UiO-66/MWCNTs/AuNPs composite electrode, which exhibited remarkable sensitivity due to the synergistic effects of metal–organic frameworks, carbon nanomaterials, and gold nanoparticles, achieving an LOD for TET of 167 nM in buffer. Fernandes-Junior et al. [5] proposed a cost-effective and eco-friendly sensor utilizing nanodiamonds and manioc starch, demonstrating the feasibility of sustainable materials in high-performance electrochemical sensing of TET in buffer, leading to an LOD of 2.0 × 10−6 M. On the other hand, Sankhla et al. [6] employed Cu-MOF functionalized screen-printed electrodes, achieving efficient tetracycline detection with an LOD of 1.007 µM in an aqueous medium with the added benefit of scalability for disposable sensor formats. Complementarily, Malecka-Baturo et al. [7] introduced a ferrocene-labeled ssDNA aptamer biosensor capable of distinguishing tetracycline derivatives, highlighting the growing role played by aptamer-based recognition elements in selective antibiotic detection. This led to an LOD of 0.16 nM in buffer and an LOD of 0.20 nM in spiked cow milk. Together, these studies illustrate the breadth of strategies, highlighting the need for low-cost, portable aptasensors.
Within the framework of low-cost and portable sensors, semiconducting platforms have been increasingly employed for the fabrication of disposable working electrodes [8]. Among these, titania stands out due to its biocompatibility, tunable bandgap properties, hydrophilicity, and cost-effectiveness [9]. Tailoring titania into mesoporous architectures substantially increases the electroactive surface area, enhancing both charge-transfer kinetics and the density of immobilized biorecognition elements, critical for high-performance biosensing. For example, a TiO2-based composite with AuNPs and MWCNTs (AuNPs/MWCNTs@TiO2) was shown [10] to provide a conductive, high-surface-area platform that facilitates stable aptamer immobilization and, consequently, elevates sensor sensitivity.
One particularly promising material is manganese-doped titanium dioxide (TiO2:Mn), which improves charge transport and surface reactivity without compromising the inherent biocompatibility of TiO2 [11]. Doping with Mn introduces mid-gap states that improve charge transport and electron transfer at the electrode–electrolyte interface, which is crucial for electrochemical sensing [12]. Mn dopants can also generate oxygen vacancies and surface defect sites that promote the stronger adsorption of aptamers and analytes, leading to higher immobilization efficiency and improved sensitivity.
This work presents an electrochemical aptasensor featuring a mesoporous TiO2:Mn screen-printed working electrode (SPE) for the detection of tetracycline (TET). In particular, SPEs excel as working electrodes because they integrate affordability, portability, flexibility, and disposability. Their customizability and ease of fabrication have revolutionized electrochemical sensing, especially for point-of-need applications in healthcare, food safety, and environmental monitoring. In this work, a well-characterized commercial DNA aptamer [13,14] is adsorbed on a TiO2:Mn working electrode so that the sensor achieves the sensitive detection of TET in an aqueous solution and demonstrates a detection limit of approximately 1 ng/mL.
2. Materials and Methods
TiO2:Mn nanoparticles were synthesized by a sol–gel route: titanium isopropoxide was hydrolyzed in isopropanol, followed by the addition of MnCl2 to achieve a doping concentration of less than 1% [15]. The gel was aged, dried, and calcined to form crystalline TiO2:Mn nanoparticles. Using a rotary evaporator, a TiO2:Mn ink was prepared and deposited onto disposable FTO (fluorine tin oxide) conductive substrates using a standard screen-printing method. The electrodes were then cured at 550 °C in a muffle furnace to produce a mesoporous thick-film TiO2:Mn coating robust enough for electrochemic al analysis, with porosity beneficial for aptamer attachment and analyte accessibility.
Tetracycline (TET; molecular weight 444.43 Da), originally derived from Streptomyces species, was prepared in spiked aqueous samples at concentrations ranging from 0.3 to 25 ng/mL. A DNA aptamer with the sequence 5′-CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG-3′ was synthesized by GeneCust (Boynes, France). The binding buffer was NaCl (100 mM), Tris–HCl (20 mM, pH 7.6), MgCl2 (2 mM), KCl (5 mM) and CaCl2 (1 mM), while 1 mM of ferro/ferricyanide [Fe(CN)6]4−/3− was added as a redox probe for electrochemical measurements. The electrodes were incubated for 2 h with 5 µM of aptamer in the prepared binding buffer for efficient adsorption onto the mesoporous TiO2:Mn surface. Post-incubation, rinsing steps removed loosely bound aptamers, ensuring a stable immobilized recognition layer. All solutions were prepared using deionized Milli-Q water, while all reagents were purchased from Sigma Aldrich-Merck (Darmstadt, Germany).
TET detection was carried out using differential pulse voltammetry (DPV) with a potential range from −0.1 V to +0.5 V and a resting time of 60 s. The electrochemical measurements were recorded by a PalmSens 4 potentiostat (PalmSens BV, Houten, The Netherlands), with a screen-printed TiO2:Mn working electrode of area 0.5 cm × 2.5 cm, as well as a Ag/AgCl reference, and a Pt wire as a counter. The Ag/AgCl reference and the Pt wire were purchased from BVT (BVT Technologies, Brno, Czech Republic). The screen-printed electrodes were prepared using a SNOL 3/1100 muffle furnace, an IKA RV 3V, (IKA, Staufen, Germany)and a Kaivo KV-SP HY210 screen printer (KV-SP HY210, Kaivo, Shangai, China). SEM was performed using a JEOL-JSM-IT 200 instrument (JEOL Ltd., Tokyo, Japan), while the Raman spectra of the TiO2:Mn samples were recorded at the 532 nm excitation mode from a diode laser using a Jobin Yvon T64000 Raman Spectrometer in the backscattered mode (HORIBA Jobin Yvon, Longjumeau, France). The presence of the aptamer on the electrode surface was investigated using SEM/EDS as above.
3. Results and Analysis
3.1. Material Characteristics
SEM visualizations (Figure 1) of the screen-printed electrodes indicated mesoporous architectures that are essential for increased surface area and aptamer loading. The EDS (Figure 2) showed the expected elemental analysis with Mn, Ti, and O and traces of Sn present from the substrate.
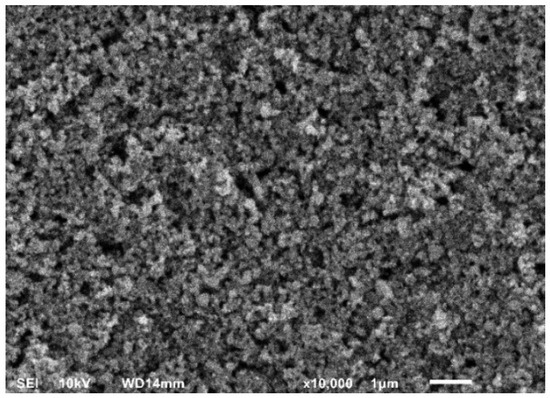
Figure 1.
SEM analysis of TiO2:Mn reveals uniform mesoporous morphology with interconnected pores and structural integrity.
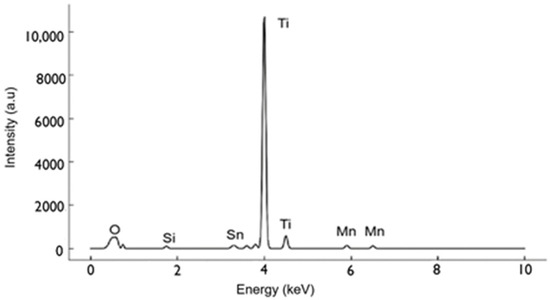
Figure 2.
EDS analysis confirms elemental composition with peaks for Ti, O, and Mn (dopant), alongside Sn signals originating from the underlying substrate.
The Raman spectra of the TiO2:Mn working electrode (Figure 3) after preparation are dominated by characteristic rutile stretching peaks at 144 cm−1, 445 cm−1, and 613 cm−1, corresponding to the B1g, Eg, and A1g symmetries, respectively [16]. In addition, the peak observed at 243 cm−1 is attributed to second-order effects, such as multiphoton scattering processes.
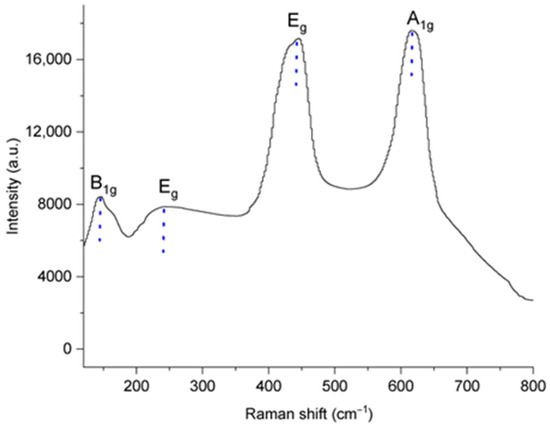
Figure 3.
Raman spectra of the TiO2:Mn sample, showing dominant rutile characteristic peaks at 144, 445, and 613 cm−1.
An energy-dispersive X-ray spectroscopy (EDS) analysis of the TiO2:Mn working electrode after the aptamer was adsorbed and washed off was conducted to obtain elemental composition information and confirm the presence of the aptamer on its surface. In particular, the spectrum obtained (Figure 4) shows that titanium and oxygen are the predominant elements, with weight percentages of 52.69% and 46.37%, respectively, consistent with the expected TiO2 stoichiometry. A small signal from manganese (0.55 wt%) verifies successful Mn incorporation into the lattice, while minor traces of tin (0.28 wt%) are also detected. The corresponding atomic percentages further confirm a Ti:O ratio close to the ideal composition of TiO2, with Mn and P present only at low concentrations.
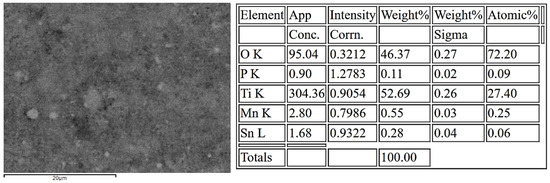
Figure 4.
EDS elemental composition of the TiO2:Mn sample after aptamer adsorption. Titanium and oxygen dominate the spectrum, while minor signals of Mn confirm doping. The phosphorus peak arises from the phosphate backbone of aptamers adsorbed on the TiO2:Mn surface.
Notably, the presence of phosphorus (0.11 wt%) can be attributed to the phosphate backbone of the aptamers adsorbed on the TiO2:Mn surface. This provides direct evidence of aptamer adsorption, considering that the phosphorus signal originates from the DNA phosphate groups bound at the electrode interface. We can thus argue that aptamers are immobilized onto the TiO2:Mn electrodes primarily through non-covalent adsorption forces. The negatively charged phosphate backbone of the DNA interacts electrostatically with positively charged Ti/Mn surface sites, while additional stabilization arises from hydrogen bonding with surface hydroxyls, van der Waals, and π–π interactions between nucleobases and oxide sites.
3.2. Detection of Tetracycline
DPV was used to observe current suppression as a function of TET concentration, using ferro/ferricyanide as a redox probe (Figure 5). The DPV signals display a well-defined oxidation peak centered around 0.2 V, with the peak current decreasing progressively as the TET concentration increases. This inverse relationship between the current and analyte concentration is typical in electrochemical sensing where the presence of the analyte suppresses the electron transfer process.
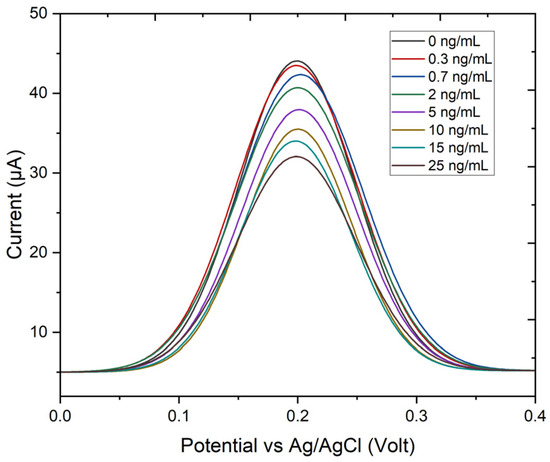
Figure 5.
This figure presents the differential pulse voltammetry (DPV) response of an electrochemical sensor to varying concentrations of tetracycline (TET), ranging from 0.3 to 25 ng/mL.
In particular, the detection mechanism of an aptamer adsorbed on the TiO2:Mn/FTO working electrode with a ferro/ferri redox probe relies on changes in interfacial electron transfer. The TiO2:Mn/FTO electrode provides a conductive, high-surface-area platform for aptamer immobilization, while the [Fe(CN)6]3−/4− couple serves as a sensitive electrochemical probe. In the absence of tetracycline, the aptamer-modified surface allows for efficient electron transfer, producing higher differential pulse voltammetry (DPV) currents. Upon tetracycline binding, the aptamer undergoes a conformational change that forms a compact aptamer–tetracycline complex, creating steric and electrostatic barriers that hinder access of the negatively charged ferro/ferri ions to the electrode. This reduces charge-transfer efficiency, leading to a concentration-dependent decrease in the DPV peak current, which serves as the analytical signal for tetracycline detection.
A linear correlation was seen between the logarithm of the TET concentration (0.3, 0.7, 2, 5, 10, 15, and 25 ng/mL) and peak current changes. The LOD was calculated using (I-Io)/Io, versus the log of the TET concentration for a linear fit (Figure 6), giving a value for the LOD of less than 1 ng/mL, which is equivalent to approximately 2.3 nM [17].
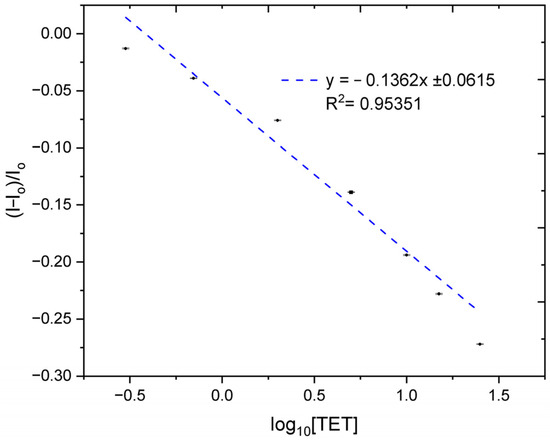
Figure 6.
This plot shows the calibration curve for the electrochemical detection of tetracycline (TET), where the normalized peak current is plotted against the logarithm of the TET concentration in ng/mL.
Thus, the porous screen-printed TiO2:Mn electrodes functionalized with aptamers achieved a detection limit of ≈2.3 nM for tetracycline, which is competitive when benchmarked against other TiO2-based sensing strategies. For instance, porous magnetic TiO2–MIP electrodes demonstrated a slightly lower LOD of 0.916 nM through the use of a molecularly imprinted polymer [18], while oxygen-vacancy-engineered TiO2 nanotubes pushed the sensitivity further to 0.33 nM by enhancing charge separation [19], albeit at the expense of complex defect engineering. In contrast, a self-powered Ti3O/R-TiO2 nanocomposite device exhibited a considerably higher LOD of 39 nM, underscoring the trade-off between bias-free operation and sensitivity [20].
In this context, porous screen-printed TiO2:Mn electrodes with aptamer adsorption offer a balanced compromise between sensitivity, fabrication simplicity, and scalability. Their performance is clearly competitive with more sophisticated TiO2-based architectures while maintaining the low-cost and practical advantages of screen-printing technology, making them highly attractive for real-world applications in antibiotic detection.
4. Conclusions
We report a cost-effective, high-performance electrochemical aptasensor employing porous TiO2:Mn screen-printed electrodes for tetracycline detection. The tailored Mn doping enhances charge transfer and surface binding, enabling dose–response detection in the nanogram-per-milliliter range. Such a platform would be suitable for food and environmental testing. Future efforts will transition the method toward real-world matrices (e.g., milk, soil, and water), handheld devices, and multiplexing capabilities for broader environmental and health monitoring.
Author Contributions
Conceptualization, L.G.B.; methodology L.G.B. and M.K.; formal analysis, L.G.B.; investigation, M.K. and L.G.B.; writing—original draft preparation, L.G.B. and M.K.; writing—review and editing, L.G.B. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
All the investigations were funded by the European Union in the framework of H2020-MSCA-RISE-2020 SAFEMILK, Project No. 101007299 under the Marie Sklodowska-Curie grant agreement.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Electrochemical DNA biosensors. Electroanalysis 2009, 21, 1237–1250. [Google Scholar] [CrossRef]
- Raykova, M.R.; Corrigan, D.K.; Holdsworth, M.; Henriquez, F.L.; Ward, A.C. Emerging Electrochemical Sensors for Real-Time Detection of Tetracyclines in Milk. Biosensors 2021, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Huang, J.; Ye, H.; Xu, H.; Cai, D.; Wang, D. A High-Performance Electrochemical Sensor for Sensitive Detection of Tetracycline Based on a Zr-UiO-66/MWCNTs/AuNPs Composite Electrode. Anal. Methods 2022, 14, 3000–3010. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Junior, W.S.; Zaccarin, L.F.; Oliveira, G.G.; de Oliveira, P.R.; Kalinke, C.; Bonacin, J.A.; Prakash, J.; Janegitz, B.C. Electrochemical Sensor Based on Nanodiamonds and Manioc Starch for Detection of Tetracycline. J. Sens. 2021, 2021, 6622612. [Google Scholar] [CrossRef]
- Sankhla, L.; Kumar, A.; Kushwah, H.S. Electrochemical Detection of Tetracycline Using Cu-MOF Functionalised Screen-Printed Electrodes. Sci. Rep. 2025, 15, 19129. [Google Scholar] [CrossRef] [PubMed]
- Malecka-Baturo, K.; Zaganiaris, A.; Grabowska, I.; Kurzątkowska-Adaszyńska, K. Electrochemical Biosensor Designed to Distinguish Tetracyclines Derivatives by ssDNA Aptamer Labelled with Ferrocene. Int. J. Mol. Sci. 2022, 23, 13785. [Google Scholar] [CrossRef] [PubMed]
- Bousiakou, L.; Al-Dosary, O.; Economou, A.; Subjakova, V.; Hianik, T. Current Trends in the Use of Semiconducting Materials for Electrochemical Aptasensing. Chemosensors 2023, 11, 438. [Google Scholar] [CrossRef]
- Amri, F.; Fatimawali; Yulizar, Y.; Pakaya, Z.; Retnaningsih, C. Mesoporous TiO2-Based Architectures as Promising Sensing Materials towards Next-Generation Biosensing Applications. RSC Adv. 2021, 11, 21477–21498. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, H.; Xie, M.; Zhao, F.; Han, S. Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on AuNPs/MWCNTs@TiO2 Nanocomposites. Int. J. Mol. Sci. 2023, 24, 10594. [Google Scholar] [CrossRef] [PubMed]
- Al Fatease, A.; Haque, M.; Umar, A.; Ansari, S.G.; Alhamhoom, Y.; Muhsinah, A.B.; Mahnashi, M.H.; Guo, W.; Ansari, Z.A. Label-Free Electrochemical Sensor Based on Pure and Mn-Doped TiO2 Nanoparticles for Myoglobin Detection: Biomarker for Acute Myocardial Infarction. Molecules 2021, 26, 4252. [Google Scholar] [CrossRef] [PubMed]
- Vallez, L.; Jimenez-Villegas, S.; Garcia-Esparza, A.T.; Jiang, Y.; Park, S.; Wu, Q.; Gill, T.M.; Sokaras, D.; Siahrostami, S.; Zheng, X. Effect of Doping TiO2 with Mn for Electrocatalytic Oxidation in Acid and Alkaline Electrolytes. RSC Adv. 2022, 12, 357–366. [Google Scholar] [CrossRef]
- Karapetis, S.; Bousiakou, L.G. Developing a Sensitive Method for the Electrochemical Determination of Tetracycline Using MB-Tagged Aptamers on Gold Electrode Substrates. Eng. Proc. 2023, 35, 38. [Google Scholar] [CrossRef]
- Niazi, J.H.; Lee, S.J.; Gu, B.M. Single-stranded DNA aptamers specific for antibiotics tetracyclines. Bioorganic Med. Chem. 2008, 16, 7245–7253. [Google Scholar] [CrossRef] [PubMed]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Sol–gel synthesis and characterization of pure and manganese doped TiO2 nanoparticles—A new NLO active material. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 120, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Podelinska, A.; Neilande, E.; Pankratova, V.; Serga, V.; Bandarenka, H.; Burko, A.; Piskunov, S.; Pankratov, V.A.; Sarakovskis, A.; Popov, A.I.; et al. Structural and Spectroscopic Characterization of TiO2 Nanocrystalline Materials Synthesized by Different Methods. Nanomaterials 2025, 15, 498. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Shao, Y.; Zheng, R.; Wang, P.; Li, Y.; Zhao, Z.; An, J.; Hao, C.; Kang, M. A novel surface molecularly imprinted polymer electrochemical sensor based on porous magnetic TiO2 for highly sensitive and selective detection of tetracycline. Environ. Sci. Nano 2023, 10, 1614–1628. [Google Scholar] [CrossRef]
- Cui, H.; Yao, C.; Cang, Y.; Liu, W.; Zhang, Z.; Miao, Y.; Xin, Y. Oxygen vacancy-regulated TiO2 nanotube photoelectrochemical sensor for highly sensitive and selective detection of tetracycline hydrochloride. Sens. Actuators B Chem. 2022, 359, 131564. [Google Scholar] [CrossRef]
- Huang, G.; Si, H.; Zou, L.; Zhang, H.; Liao, J.; Lin, S. Phase Modulation of Ti3O/R-TiO2 Nanocomposites for the Self-Powered Photoelectrochemical Sensing of Tetracycline. ACS Appl. Nano Mater. 2024, 7, 715–725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).