1. Introduction
Kapton-H is a high-performance thermoplastic polymer, well-known for its high thermal stability, great electrical/thermal insulation capability, good resistance to ionizing radiation (e.g., X-rays, protons), etc. [
1]. However, Kapton-H is also an optical-grade plastic [
2]; indeed, it is a perfectly transparent organic solid [
3] with a refractive index value quite close to that of silica glass (ca. 1.70, sodium D-line, 589.0 nm). Kapton-H’s high optical transparency is due to its semi-crystalline nature characterized by very small sized crystallites (see
Figure S1) [
4,
5] (only a partial alignment of polymer chain folding is present) that makes this polymer completely unable to scatter visible light. Kapton-H has two important functional properties that make it a very attractive material for industrial applications. Firstly, Kapton-H films uniformly absorb most of the ultraviolet spectral region [
6]—that is, the electromagnetic radiations with a wavelength ranging from 190 nm to 400 nm. Therefore, it can be used as a shield for this type of ionizing radiation (specifically, radiations belonging to the UV-A, -B, and -C spectral sub-bands are mostly extinguished). Since Kapton-H is a high-performance thermoplastic polymer widely used in space technology [
7], additional shielding characteristics further expand the advantages deriving from its use in this special area of technology (UV radiation is particularly abundant outside the earth atmosphere). In addition, this optical plastic strongly absorbs visible light in the 400–500 nm spectral range (i.e., violet, blue, and green lights). Such strong optical absorption causes the characteristic bright gold-yellow colouration shown by this plastic material [
8] which, consequently, can be industrially exploited for applications such as the colour filter [
9]. In particular, yellow colour filters are very useful optical devices since they have a number of industrial applications, varying from pharmaceutical packaging to snow/night/sport goggles, safety lighting systems, car headlights, and/or fog lights, etc. [
10,
11].
The photo-excitation of Kapton-H in ultraviolet/visible spectral regions can be accurately described using the band theory [
2]. This solid-state physical theory can be indifferently applied to all type of covalent crystals and amorphous solids, including polymers, glasses, and other dielectric substances [
12,
13,
14]. The special absorption behaviour of Kapton-H is a consequence of the interband electronic transitions. In particular, photons have the capability of exciting electrons from whatever occupied levels in the valence band (VB) to whatever unoccupied levels in the conduction band (CB). The band edge transition is named HOMO-LUMO transition. This interband transition is purely quantum mechanical in nature; therefore, the transition is quantized. However, energetic levels in both bands are so close (ca. 10
−22 eV) that the photon absorption appears like a continuum phenomenon is taking place when the band edge energy is exceeded.
Here, the absorption properties of Kapton-H in the UV-Vis-NIR spectral regions have been investigated in-depth using optical spectroscopy measurements combined with standard analysis methods such as the Tauc plot [
15], Urbach rule [
16], Cody plot [
17], etc. In particular, these analyses have allowed us to evaluate Kapton-H electronic/optical characteristics such as band gap energy (E
g), Urbach energy (E
U), visible transparency percentage, and cut-on wavelength, as well as allowing us to quantify the structural disorder in this amorphous material.
2. Materials and Methods
Kapton-H films were provided by Wetec (GmbH, Germany) and polyetherimide (PEI) pellets were provided by Aldrich (St. Louis, MO, USA). Kapton-H (i.e., poly-oxydiphenylene-pyromellitimide, PMDA-ODA, -(C22H10N2O2)n-) is a linear polymer with heterocyclic rings that are linked together by one or more covalent bonds. Optical spectra were obtained at room temperature using a double beam UV/Visible spectrophotometer (VWR, UV-6300PC Spectrophotometer, VWR International Europe bvba, Leuven, Belgium). These spectra were recorded in the 190–1100 nm wavelength range with a low scan speed and a scan step (resolution) of 0.5 nm (the slit 0.0 was used). The spectra were processed by a devoted analysis software (UV-Vis Analyst, Version 5.44). In particular, Kapton-H was tested in the form of films (rectangular specimens, 1 cm × 3 cm), without using any type of reference sample. These rectangular films were stuck to the instrument sample holder by paper adhesive tape. Thin PEI films were obtained by solution casting technology, using chloroform (Aldrich, St. Louis, MO, USA) as a solvent. Film thickness for absorption coefficient determination was measured using a digital precision micrometer (Mitutoyo, Kawasaki, Japan), giving results of 44 μm for Kapton-H and 240 μm for PEI.
3. Results
Optical spectra of Kapton-H films have been obtained in both absorbance (A) and transmittance (T) modes in the 190–1100 nm spectral range (see
Figure 1a,b). The optical spectrum is characterized by a very sharp transmittance change in the visible light spectral range (precisely, the green-yellow region). In particular, this rapid variation in the visible light transmittance value takes place at a wavelength of ca. 550 nm; indeed, Kapton film transmittance is close to 80% at wavelengths higher than 600 nm, and close to 0% at wavelengths lower than 500 nm. Therefore, the transmittance switching can be assumed to take place at a wavelength value of ca. 550 nm. It must be pointed out that at wavelengths lower than 500 nm, the transmittance value assumes a continuous value very close to zero, up to the extreme in air spectroscopically measurable spectral value (190 nm). Therefore, Kapton should have a very broad absorption band characterized by a large extinction coefficient in the ultraviolet spectral region and consequently, absorption measurements made on this polymer only record the absorption edge. Since the transmittance change starts at 600 nm and completes at 500 nm, it is possible to assume a cut-on (or cutoff) wavelength of 550 nm for the Kapton-H optical filter. In particular, cut-on is defined as the wavelength where the absorption coefficient is 50 cm
−1.
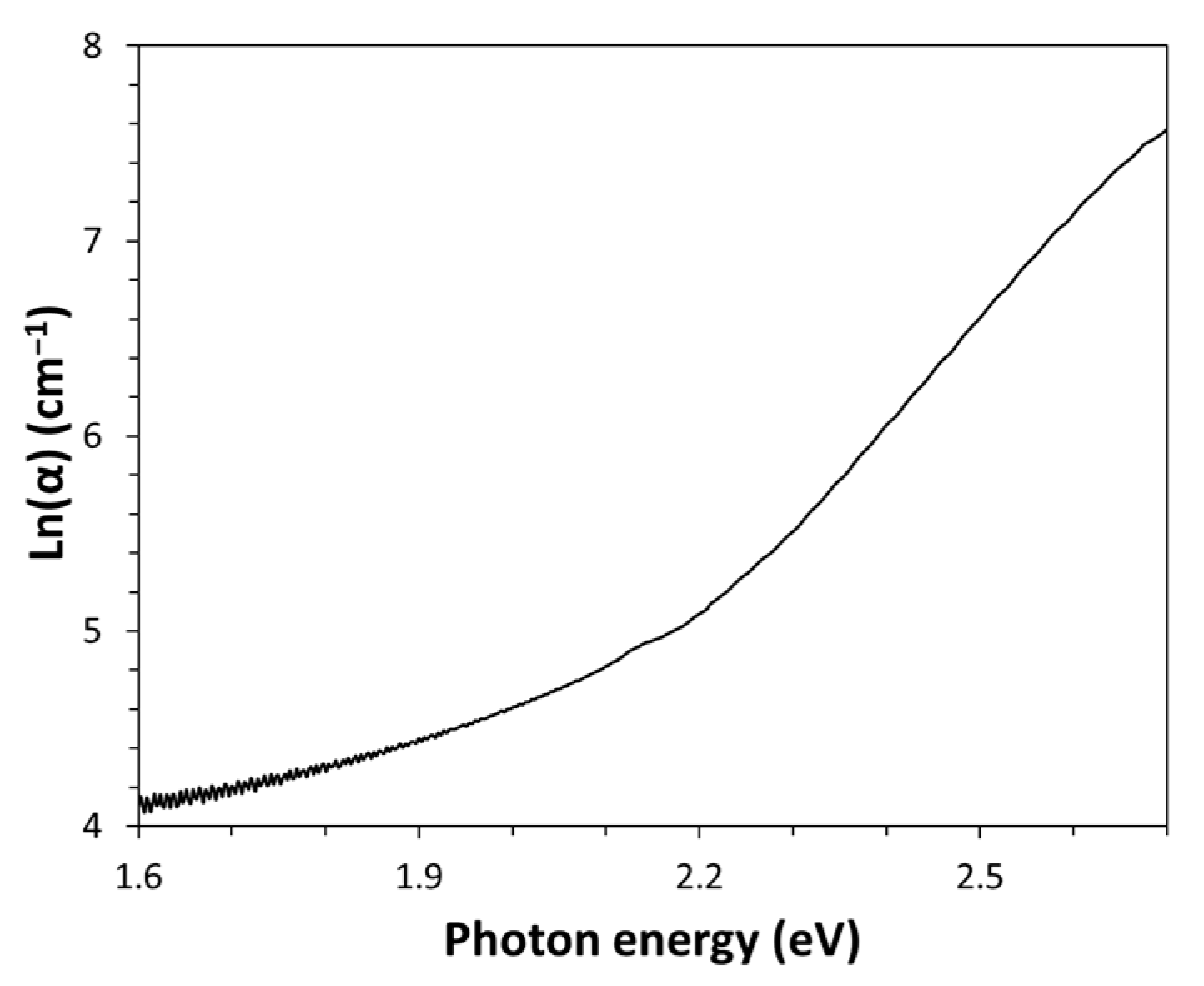
The absorption coefficient, α (i.e., fractional attenuation in intensity per unit distance) has been calculated from the raw absorbance data, A, using the law α = 2.303∙A/d [
18], where d is the path length of light, corresponding to the film thickness (d = 0.044 mm). The wavelengths corresponding to each absorption coefficient value have been converted to energy (expressed in eV) using the following equation: E(eV) = 1239.8/λ(nm) [
16]. A plot of absorption coefficient versus energy in the semi-logarithmic scale has been made (see
Figure 2). This type of spectrum representation has been used to estimate the Urbach energy value from the slope of the linear part of the spectrum. Indeed, according to the Urbach rule, the absorption coefficient (α) grows exponentially with the increasing photon energy (E):
which in logarithm form becomes
Therefore, the inverse of the slope corresponds precisely to the Urbach energy (E
U) values of Kapton-H expressed in eV; this quantity is directly proportional to the structural disorder in this amorphous polymer. A value of (185 ± 2) meV has been found by the regression analysis of the linear part of the spectrum (R
2 = 0.9996) shown in
Figure 2; such high Urbach energy indicates a strongly disordered structure of the amorphous phase in the polymer, probably due to its steric rigidity.
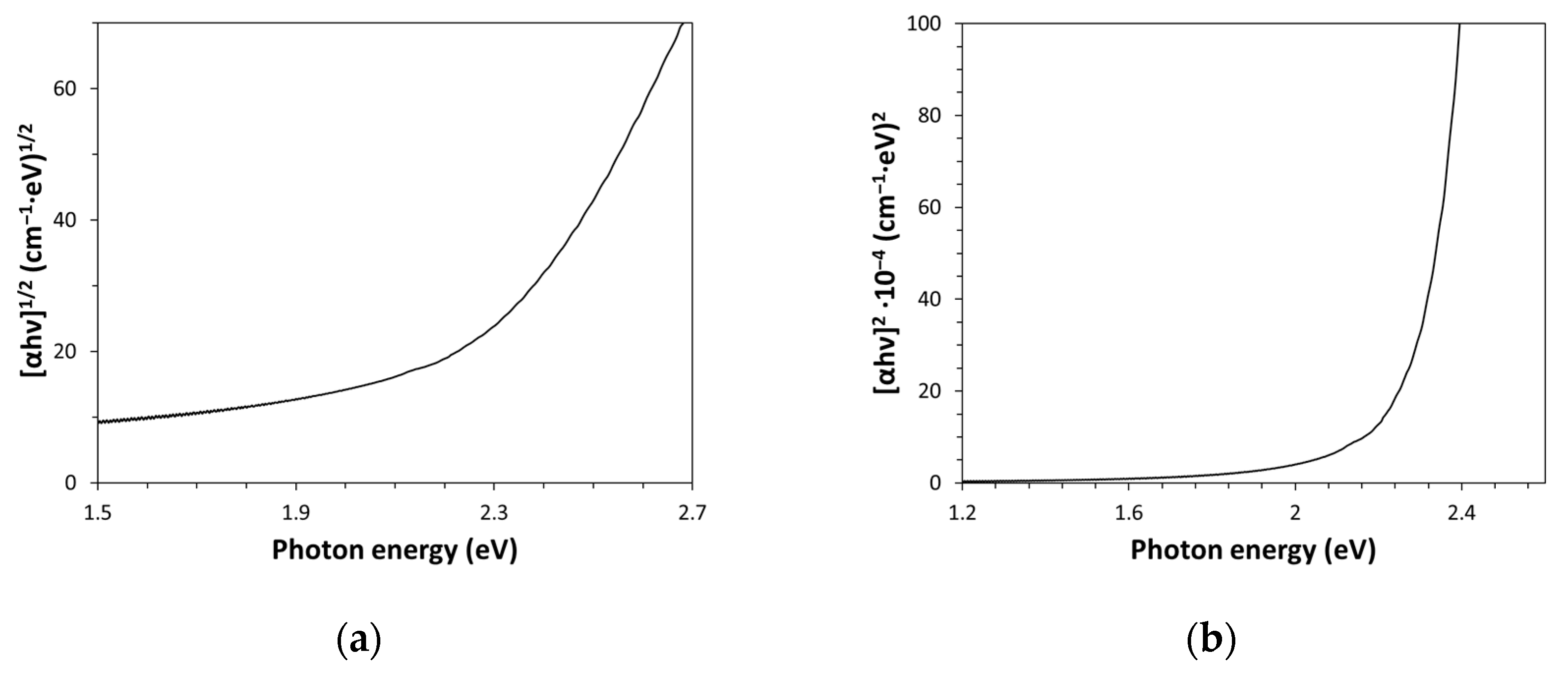
The band gap energy (E
g) value is a further important physical property of Kapton-H that can be optically estimated. Measurement of the band gap energy is essential in both the semiconductor and nanomaterial industries; however, it can also be useful in the case of dielectric bulk substances like polymers and glasses since this quantity defines the electrical and thermal insulation capability of the material. This quantity can be accurately evaluated by drawing the Tauc plot for a direct and indirect allowed transition model, with the aim to establish which model provides a better fitting of the experimental optical data. According to the Tauc plots shown in
Figure 3a,b, data were more conveniently described by an indirect allowed band gap transition model (i.e., (αhν)
1/2 = k(hν − E
g)), which is the classical expression used by Tauc for the amorphous semiconductors. This indirect allowed transition model has provided an E
g value of (2.22 ± 0.05) eV (intercept of the linear part of the graph characterized by R
2 = 0.9993, obtained as opposite of the ratio between intercept and slope, -Y(intercept)/Slope), which is in accordance with the value given in the literature for this type of amorphous polymer [
2]. The optical measurement of the band gap energy can also be exploited for analytical uses, like the identification of polymers of unknown nature, by comparing their E
g values with the data given in the literature for the different polymers.
Alternatively, the band gap energy of Kapton-H can be determined by elaborating the optical absorption measurements according to the Cody plot method [
17]. The Cody model is based on a constant dipole matrix approximation, and it is related to the absorption coefficient through the following expression: (α/hν)
2 = k(hν − E
g). As shown in
Figure 4, this plot shows linear behaviour in the high-photon-energy range (R
2 = 0.9975). This approach provides a value of E
g of (2.33 ± 0.05) eV, which is slightly higher than that estimated by the Tauc plot for the indirect transition model and therefore close to the value usually assigned this polymer [
2].
Finally, the Kapton-H optical spectrum exhibits quite standard behaviour, which includes a dramatic transmittance change from a very high value (ca. 80%) to zero at the cut-on wavelength. Compared to the typical solid polymer spectral behaviour, Kapton-H has the following two peculiarities: (i) the cut-on is located just in the middle of the visible spectral range, i.e., at ca. 550 nm, while such a transition is typically observed in the UV-A/B region for other types of polymer, and (ii) a pronounced exponential decay (i.e., the so-called Urbach tailing) is visible in the region of the fundamental absorption edge. Owing to the quite sterically rigid structure, short-range order in this amorphous polymer should be much lower than in polymers with a flexible backbone.
Kapton-H belongs to the polymeric class of polyimides; however, it is characterized by a band gap energy value that is slightly lower than that of other polyimides. Such behaviour is probably due to the much more extended conjugation in the contained aromatic heterocyclic groups and to the involved light absorption mechanism based on electron transfer [
19]. For example, according to the results obtained by applying the Tauc plot method, for an indirect allowed electron transition model, to polyetherimide (PEI) (see
Figure 5a,b), a band gap value of (2.99 ± 0.02) eV has been found. In this type of analysis, Cody plot and other electron transition models (e.g., direct allowed electron transition) do not seem to be adequate for describing electronic transition in PEI; indeed, they do not show a linearity region. The Urbach rule applied to PEI (see
Figure 5c) leads to an Urbach energy value of (78.9 ± 0.5) meV, thus indicating a lower content of structural disorder compared to Kapton-H. This lower structural disorder content should be related to the much higher PEI backbone flexibility (i.e., lower macromolecular chain stiffness). As visible in
Figure 5a, PEI has a cut-on located at a wavelength lower than that of Kapton-H (ca. 400 nm); however, it is pale-yellow in colour since its optical absorption starts in the blue spectral region. PEI is an amorphous polymer; therefore, it has quite a high visible transparency (ca. 80%), comparable to that of the semi-crystalline Kapton-H.
The technological potentialities of polymers are related to their physical properties, and some of them are strictly dependent on the values of these optically measured parameters. What is particularly useful about the characterization of amorphous part of polymers is the determination of the Urbach energy value. Indeed, in semi-crystalline polymers, XRD measurements allow us to establish only the fraction of amorphous phase in the solid—usually, a low-crystalline fraction characterizes most semi-crystalline polymers like Kapton-H (see
Figure S1). Conversely, most optical plastics are fully amorphous phases; consequently, the XRD technique cannot provide useful information about them. Structural disorder in the Kapton-H or PEI amorphous phases cannot be assessed by XRD since the diffuse halo shape of these two polymers is not related to it. However, the nature of this amorphous phase changes significantly between different amorphous polymers; indeed, chain rearrangement capability depends on the polymer flexibility, and it may vary significantly. Highly stereorigid aromatic polymers, like Kapton-H, do not have the possibility to reorganize their own macromolecular segments to increase the intermolecular interactions. The flexibility of PEI chains is much higher than Kapton-H; therefore, these polymeric chains can better organize the lowering of the system entropy. Optical measurements can be indifferently applied to all types of polymers in the form of thin films in order to obtain information on structural disorders in the amorphous phase.
4. Discussion
The optical investigation of the Kapton-H electronic structure is useful for different reasons. For example, this investigation may allow us (i) to establish the technological potentialities of such types of polymers in fields such as optics (e.g., colour filters, UV-blocking optical windows, etc.); (ii) to identify an unknown polymer sample based on its characteristic band gap energy value; and (iii) to acquire in-depth structural information on it (e.g., structural disorder content). The electronic structure of a polymer can be determined by investigating its capability to interact with photons of different wavelengths. In particular, absorption measurements in different spectral regions are required in order to obtain information on the full electronic structure of the polymer. Here, the optical spectrophotometer has allowed us to measure Kapton’s capability to absorb photons of low energy (near-infrared radiation, NIR), medium energy (visible radiation) and high energy (ultraviolet radiation, belonging to the A, B and C sub-bands). Such photon absorption is a direct consequence of the electronic transitions that can occur in the solid phase; therefore, in turn, the absorption measurement allows deducting the external (valence) electronic configuration of the polymer. According to the achieved optical spectra, Kapton-H, like other solid polymers, has a band electronic structure that contains wide spatial regions composed of energetically very close levels (delocalized orbitals) that can be both filled or unfilled by electrons (valence and conduction bands, respectively). The measurement of absorbed photon energy allows to establish both the spacing (i.e., band edge energy corresponding to the HOMO-LUMO transition) and extension of these bands.
It must be pointed out that absorption spectroscopy constitutes a universal characterization technique for polymers; indeed, optical spectra can be obtained for both amorphous and semi-crystalline polymers by using adequately thin films. As a consequence, the optical measurement of the band gap energy value can be used as a simple analytical approach to establish the nature of an unknown polymeric sample; indeed, each polymer has its own specific value of Eg.
On the other hand, Kapton optical absorption for energies below the fundamental absorption edge (Urbach region) is exponential in nature; this behaviour can be investigated in-depth, and the bending can be quantitatively described by the Urbach energy value, which reflects the amount of local intermediate states and therefore the level of structural disorder characterizing the polymeric system. Such information coming from this simple optical analysis of the amorphous polymer electronic structure makes it possible to classify amorphous polymers based on their disorder content. Finally, in the case of Kapton-H, this investigation has evidenced a high disorder content in the amorphous phase, which is compatible with a high stereorigid polymer.