Soft Wearable Patch for Continuous Cardiac Biometric Security †
Abstract
:1. Introduction
2. Materials and Methods
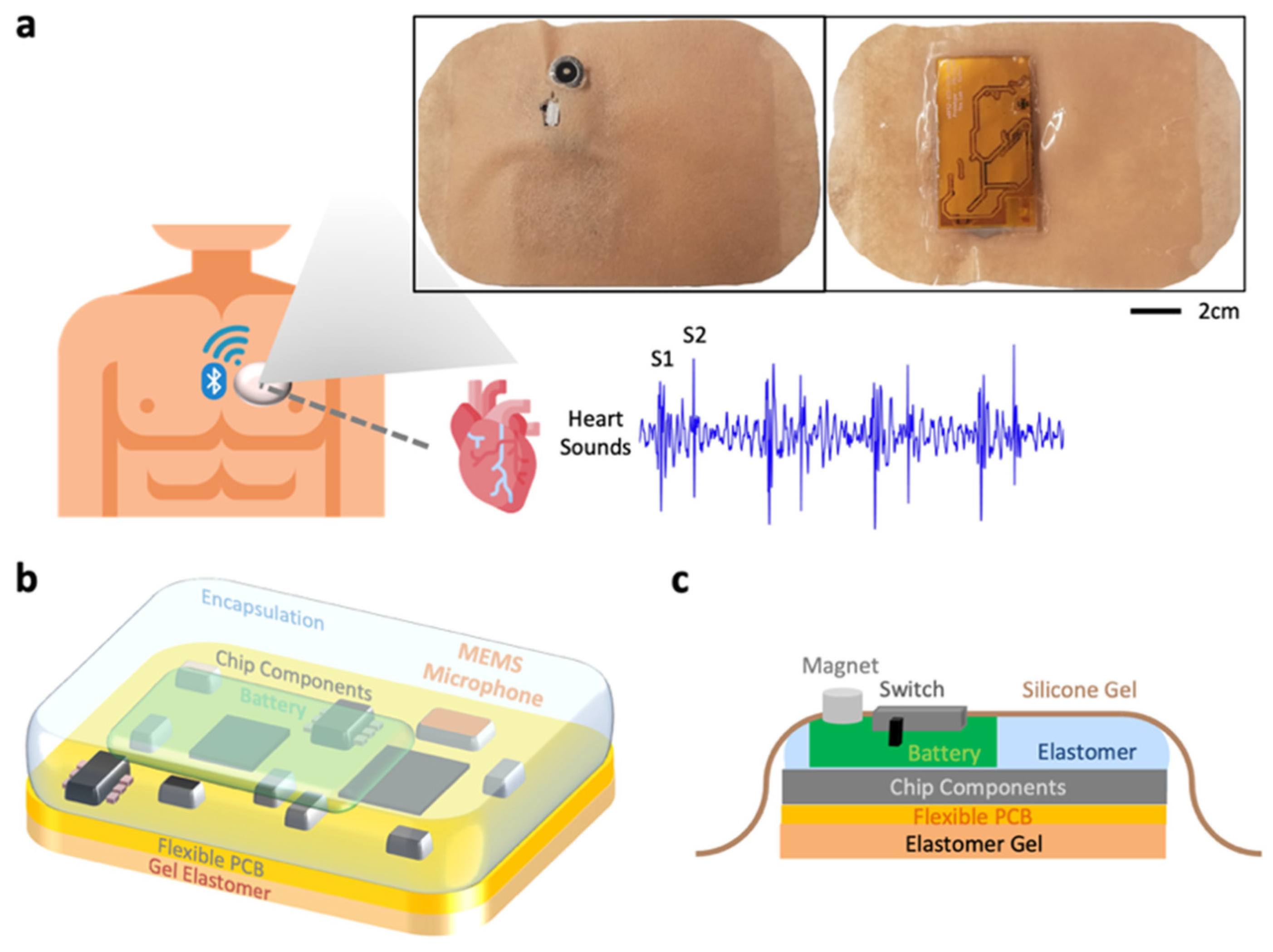
2.1. Proposed Continuous Cardiac Biometric System
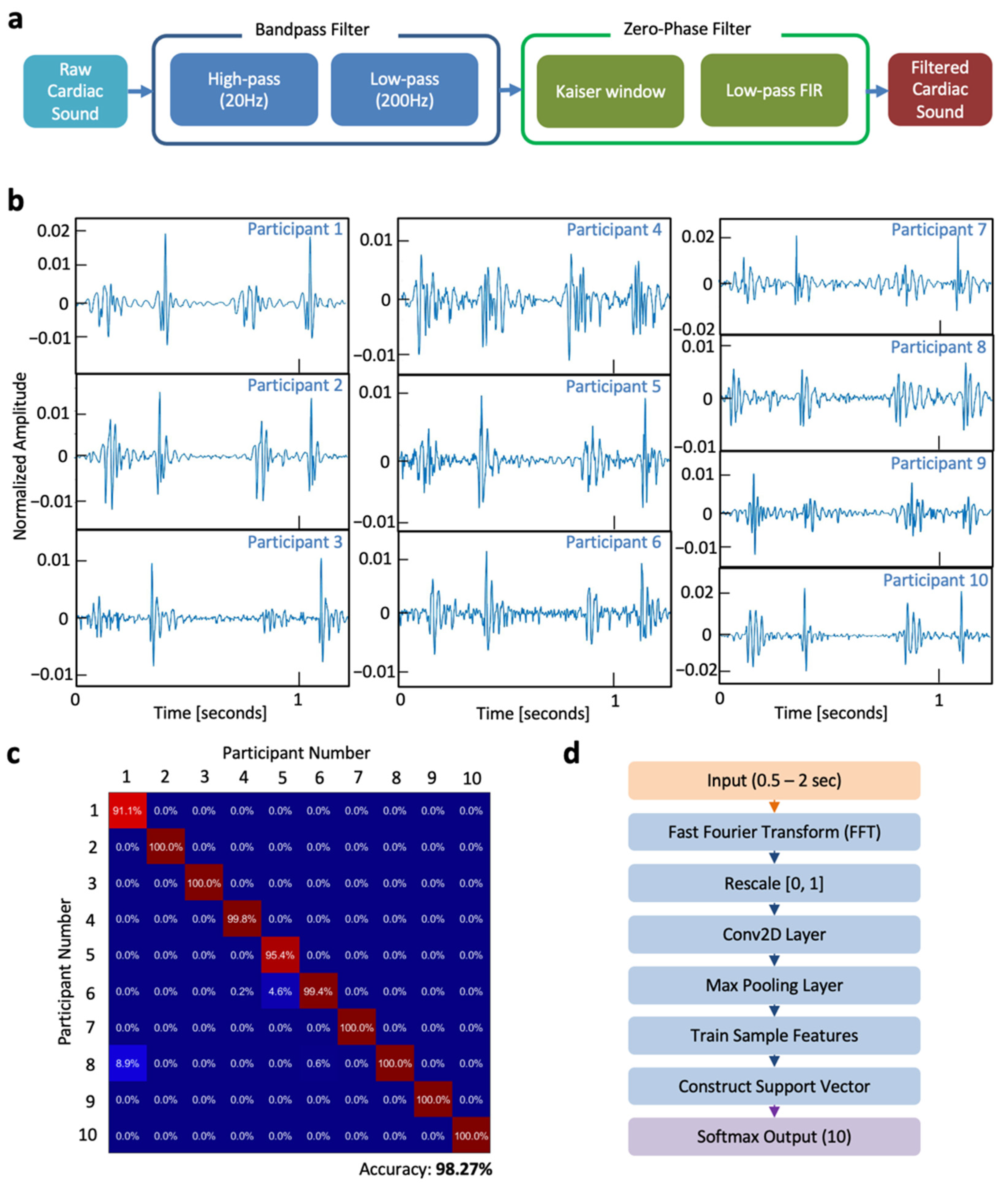
2.2. Signal Processing and Feature Extraction
3. Results and Discussion
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Li, F.; Tang, S.; Xiong, W. A Review of Computer-Aided Heart Sound Detection Techniques. BioMed Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.-S.; Yeo, W.-H. Advances in Microsensors and Wearable Bioelectronics for Digital Stethoscopes in Health Monitoring and Disease Diagnosis. Adv. Healthc. Mater. 2021, 10, 2101400. [Google Scholar] [CrossRef] [PubMed]
- Beritelli, F.; Serrano, S. Biometric Identification Based on Frequency Analysis of Cardiac Sounds. IEEE Trans. Inf. Forensics Secur. 2007, 2, 596–604. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, Q.; Ren, F. Heart Sound Biometric System Based on Marginal Spectrum Analysis. Sensors 2013, 13, 2530–2551. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Garcia, J.; Bigun, J.; Reynolds, D.; Gonzalez-Rodriguez, J. Authentication gets personal with biometrics. IEEE Signal Process. Mag. 2004, 21, 50–62. [Google Scholar] [CrossRef]
- Young, C.S. Chapter 14-Physical Security Controls. In Information Security Science; Young, C.S., Ed.; Syngress: Oxford, UK, 2016; pp. 317–338. [Google Scholar]
- Phua, K.; Chen, J.; Dat, T.H.; Shue, L. Heart sound as a biometric. Pattern Recognit. 2008, 41, 906–919. [Google Scholar] [CrossRef]
- Humayun, A.I.; Ghaffarzadegan, S.; Feng, Z.; Hasan, T. Learning Front-end Filter-bank Parameters using Convolutional Neural Networks for Abnormal Heart Sound Detection. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018. [Google Scholar]
- Kolb, F.; Spanke, J.; Winkelmann, A. Auf den Spuren des Erb’schen Auskultationspunkts: Rätsel gelöst. DMW-Dtsch. Med. Wochenschr. 2018, 143, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
| Biometric | Continuous Verification | Estimated Error Rate | Replicability | Permanence * | Sensor Type | Cost |
|---|---|---|---|---|---|---|
| This Work (Heart) | Yes | 1.7% | No | 4 | MEMS microphone | Low (<USD 5) |
| Fingerprint | No | 5.0% | Yes | 3 | Optical, ultrasound, and multispectral image | Medium (>USD 50) |
| Signature Recognition | No | 2.0% | Yes | 1 | Digitizing tablets using electromagnetic transduction | Medium (>USD 100) |
| Hand Geometry | No | 0.2% | Yes | 3 | CCD (charge-coupled device) camera | High (>USD 1000) |
| Face Geometry | No | Not Specified | Yes | 3 | High-resolution cameras, thermal sensors | High (>1000) |
| Voice Recognition | No | 2.0% | Yes | 2 | Acoustic sensors (microphones), non-acoustic sensors (electromagnetic motion sensor) | Medium (>USD 50) |
| Ear Shape | No | Not Specified | Yes | 3 | High-resolution camera and 3D imaging | Not Specified |
| Retina | No | 0.00001% | Yes | 4 | Scanner using infrared light | High (>USD 1000) |
| Iris | No | 0.0008% | Yes | 4 | Basic camera using infrared light | Medium (>USD 100) |
| Palm Veins | No | 0.88% | Yes | 3 | Infrared light | Medium (>USD 200) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Kim, Y.-S.; Yeo, W.-H. Soft Wearable Patch for Continuous Cardiac Biometric Security. Eng. Proc. 2021, 10, 73. https://doi.org/10.3390/ecsa-8-11336
Lee SH, Kim Y-S, Yeo W-H. Soft Wearable Patch for Continuous Cardiac Biometric Security. Engineering Proceedings. 2021; 10(1):73. https://doi.org/10.3390/ecsa-8-11336
Chicago/Turabian StyleLee, Sung Hoon, Yun-Soung Kim, and Woon-Hong Yeo. 2021. "Soft Wearable Patch for Continuous Cardiac Biometric Security" Engineering Proceedings 10, no. 1: 73. https://doi.org/10.3390/ecsa-8-11336
APA StyleLee, S. H., Kim, Y.-S., & Yeo, W.-H. (2021). Soft Wearable Patch for Continuous Cardiac Biometric Security. Engineering Proceedings, 10(1), 73. https://doi.org/10.3390/ecsa-8-11336








