A New Approach for Monitoring Sweat Ammonia Levels Using a Ventilated Capsule †
Abstract
:1. Introduction
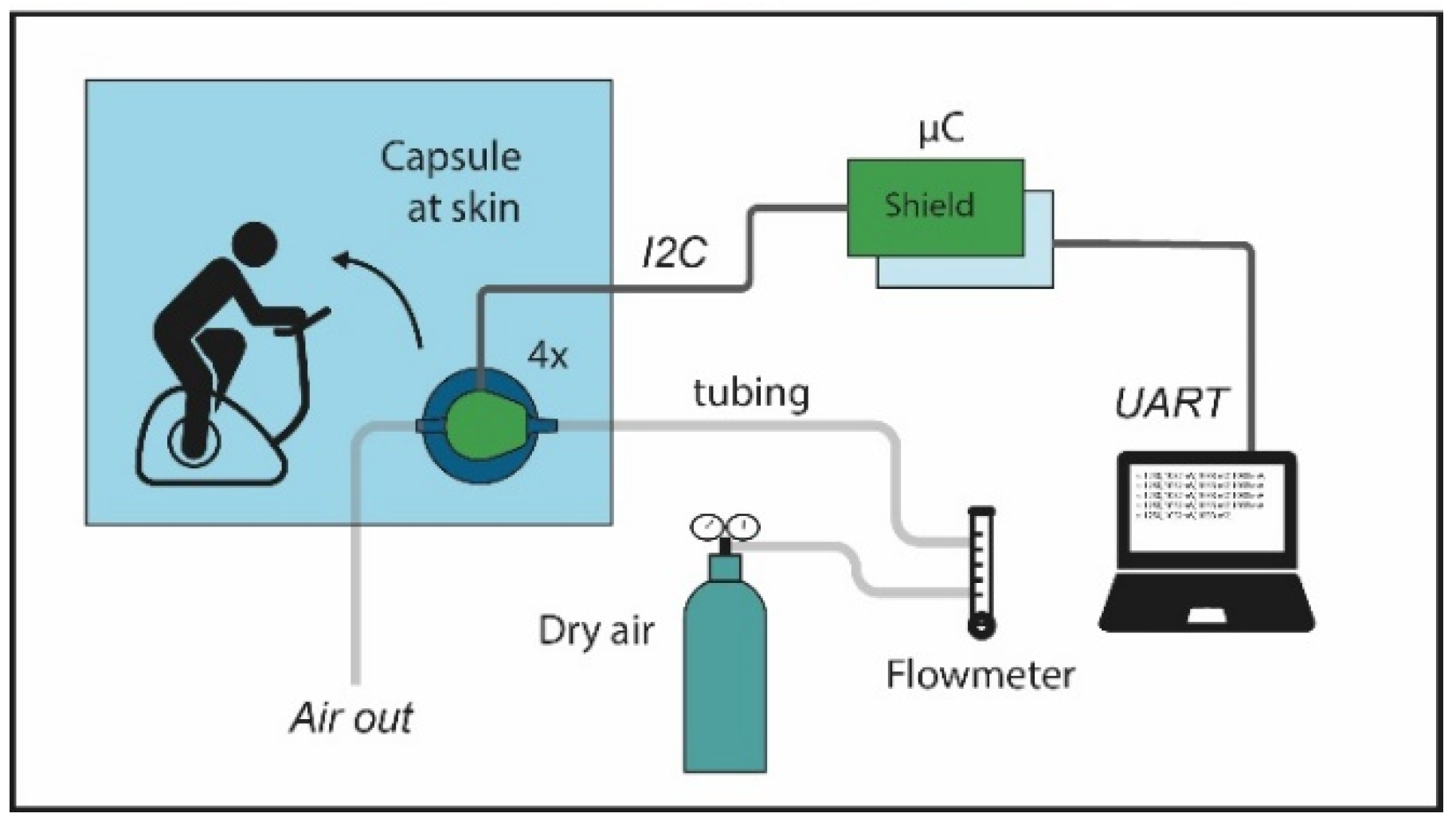
2. Method
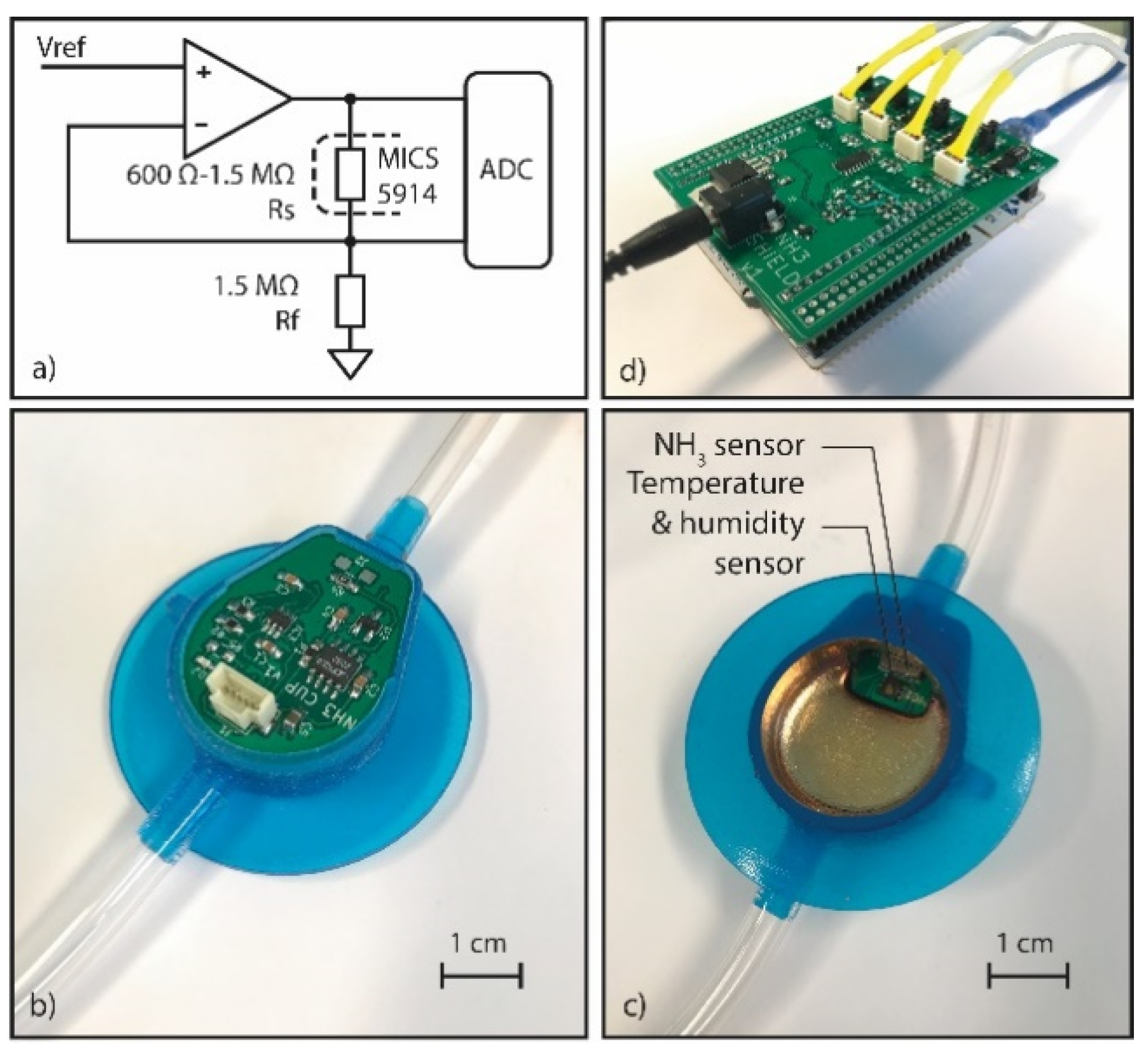
2.1. Design
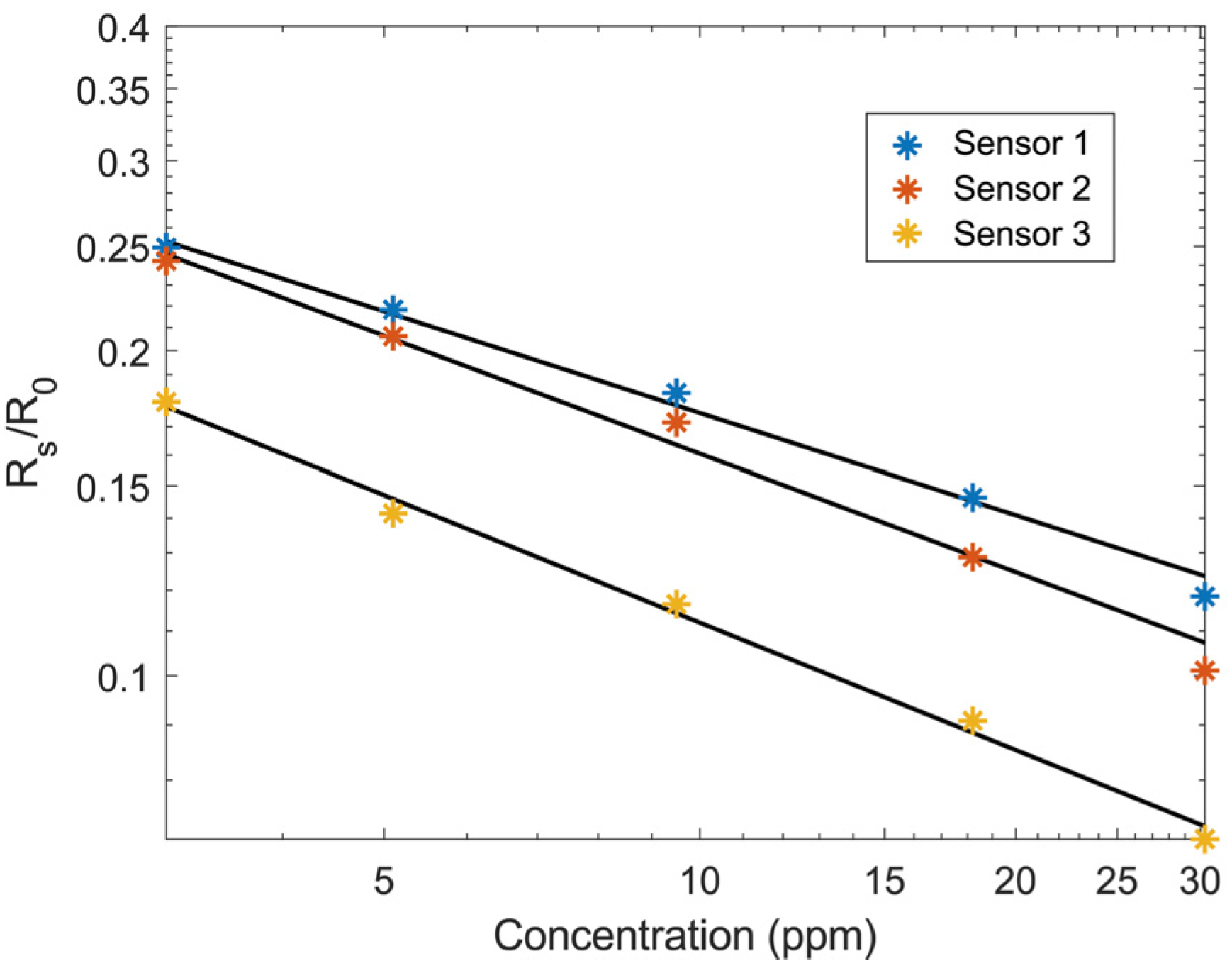
2.2. Sensor Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, J.S.; McLellan, T.H. The Transition from Aerobic to Anaerobic Metabolism. Res. Q. Exerc. Sport 1980, 51, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, P.J.; Barr, H.; Davis, F.; Higson, S.P.J. Lactate in human sweat: A critical review of research to the present day. J. Physiol. Sci. 2012, 62, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Klous, L.; de Ruiter, C.J.; Scherrer, S.; Gerrett, N.; Daanen, H.A.M. The (in)dependency of blood and sweat sodium, chloride, potassium, ammonia, lactate and glucose concentrations during submaximal exercise. Eur. J. Appl. Physiol. 2020, 121, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Alvear-Ordenes, I.; Garcia-Lopez, D.; De Paz, J.A.; Gonzalez-Gallego, J. Sweat lactate, ammonia, and urea in rugby players. Int. J. Sports Med. 2005, 26, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, F.J.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.; LeBouf, R.F.; Stefaniak, A.B. Formulation and stability of a novel artificial human sweat under conditions of storage and use. Toxicol. In Vitro 2010, 24, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, N.V.; Ha, C.-E. Contractile Systems. In Essentials of Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2011; pp. 241–259. [Google Scholar]
- Noury, J.-B.; Zagnoli, F.; Carré, J.-L.; Drouillard, I.; Petit, F.; Le Maréchal, C.; Marcorelles, P.; Rannou, F. Exercise testing-based algorithms to diagnose McArdle disease and MAD defects. Acta Neurol. Scand. 2018, 138, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Sinkeler, S.P.T.; Daanen, H.A.M.; Wevers, R.A.; Oei, T.L.; Joosten, E.M.G.; Binkhorst, R.A. The relation between blood lactate and ammonia in ischemic handgrip exercise. Muscle Nerve 1985, 8, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Zoerner, A.; Oertel, S.; Jank, M.P.M.; Frey, L.; Langenstein, B.; Bertsch, T. Human Sweat Analysis Using a Portable Device Based on a Screen-printed Electrolyte Sensor. Electroanalysis 2018, 30, 665–671. [Google Scholar] [CrossRef]
- Morris, N.B.; Cramer, M.N.; Hodder, S.G.; Havenith, G.; Jay, O. A comparison between the technical absorbent and ventilated capsule methods for measuring local sweat rate. J. Appl. Physiol. 2013, 114, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krivec, M.; Gunnigle, G.M.; Abram, A.; Maier, D.; Waldner, R.; Gostner, J.M.; Überall, F.; Leitner, R. Quantitative Ethylene Measurements with MOx Chemiresistive Sensors at Different Relative Air Humidities. Sensors 2015, 15, 28088–28098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 3M. Technical Information Sheet Product Number #1522 3MTM Double Coated Medical Tape. Available online: https://multimedia.3m.com/mws/media/792049O/3m-1522-dc-polyethylene-tape-tis-jul13.pdf (accessed on 6 December 2019).
- SGXSensortech. SGX Metal Oxide Gas Sensors. Available online: https://nl.mouser.com/pdfdocs/AN-0172-SGX-Metal-Oxide-Gas-Sensors-V1.pdf (accessed on 2 June 2021).
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steijlen, A.; Bastemeijer, J.; Nederhoff, R.; Jansen, K.; French, P.; Bossche, A. A New Approach for Monitoring Sweat Ammonia Levels Using a Ventilated Capsule. Eng. Proc. 2021, 10, 38. https://doi.org/10.3390/ecsa-8-11332
Steijlen A, Bastemeijer J, Nederhoff R, Jansen K, French P, Bossche A. A New Approach for Monitoring Sweat Ammonia Levels Using a Ventilated Capsule. Engineering Proceedings. 2021; 10(1):38. https://doi.org/10.3390/ecsa-8-11332
Chicago/Turabian StyleSteijlen, Annemarijn, Jeroen Bastemeijer, Robbert Nederhoff, Kaspar Jansen, Paddy French, and Andre Bossche. 2021. "A New Approach for Monitoring Sweat Ammonia Levels Using a Ventilated Capsule" Engineering Proceedings 10, no. 1: 38. https://doi.org/10.3390/ecsa-8-11332
APA StyleSteijlen, A., Bastemeijer, J., Nederhoff, R., Jansen, K., French, P., & Bossche, A. (2021). A New Approach for Monitoring Sweat Ammonia Levels Using a Ventilated Capsule. Engineering Proceedings, 10(1), 38. https://doi.org/10.3390/ecsa-8-11332






