Abstract
N,N-Dimethyl-4-amino-2,1,3-benzothiadiazole (BTDNMe2) was synthesized from the commercially available 2,1,3-benzothiadiazole (BTD) by nitration in a sulfonitric mixture, followed by a reduction of the nitro group and subsequent methylation with iodomethane. BTDNMe2 was fully characterized by means of nuclear magnetic resonance (NMR) and infrared spectroscopy. The solutions of BTDNMe2 in common organic solvents revealed to be appreciably luminescent in the visible range. The electronic transitions related to the absorption and emission properties were associated with the HOMO–LUMO energy gap by means of electrochemical measurements and DFT calculations. Finally, BTDNMe2 was successfully used to prepare luminescent-doped poly(methyl methacrylate) samples.
1. Introduction
2,1,3-Benzothiadiazole (BTD) is a π-extended heteroarene used for a wide range of applications such as herbicide, fungicide, and antibacterial agents [1]. However, it is also applied for the preparation of luminescent materials owing to the strong withdrawing ability and behaviour as a fluorophore [2]. Intermolecular interactions such as heteroatom contacts and π–π stacking determine well-ordered structures [3]. Over the last decades, polymers [4] and liquid crystals [5] containing BTD fragments were successfully exploited for advanced applications [6], such as organic light-emitting diodes (OLEDs) [7,8], dyes [9,10,11,12,13,14], and solar and photovoltaic cells [15,16,17,18]. 2,1,3Benzothiadiazole-based compounds were also exploited as fluorescent probes for bioimaging in live cells [19,20,21] or as fluorescent polymeric thermometers for the determination of intercellular temperature [22,23]. Moreover, poly(benzothiadiazoles) were used as heterogeneous photocatalysts to promote various organic photoredox reactions under visible-light irradiation [24,25].
Despite the fact that 4-amino-2,1,3-benzothiadiazole was deeply investigated both as a free compound [26] and a possible ligand [27,28,29,30,31,32,33] for the preparation of transition metal complexes, N,N-dimethyl-4-amino-2,1,3-benzothiadiazole (BTDNMe2) is much less studied. The only preparation reported in literature dates back to 1976 [34], but the compound was only poorly characterized. Herein, we report an alternative synthesis and the characterization of BTDNMe2, with a particular interest in the photophysical properties of the compound. The possible application of BTDNMe2 as a dopant for polymeric materials was also explored.
2. Materials and Methods
Commercial solvents (Merck) were purified following standard methods [35]. 2,1,3-Benzothiadiazole and the other reagents were Aldrich products, which were used as received. Poly(methyl methacrylate) (PMMA, Mw = 86,000 g mol−1) was a TCI Chemicals product. 4-Nitro-2,1,3-benzothiadiazole (BTDNO2) was synthesized by modifying a reported procedure [36]. An amount of 24 mL of H2SO4 98% and 8 mL of HNO3 70% were mixed in a flask and frozen with a nitrogen bath. 2,1,3-Benzothiadiazole (2.000 g, 14.7 mmol) was added, then the reaction was allowed to warm up at room temperature and stirred for three hours. The reaction mixture was then cooled with an ice bath, and water (15 mL) was slowly added. Subsequently, a solution containing about 18.0 g of NaOH in 40 mL of water was added within an hour. After removal from the ice bath, NaHCO3 was added in small amounts until a neutral pH was reached. The product was extracted with 2 × 40 mL of dichloromethane, and the organic fraction was washed with water (2 × 20 mL), dried over Na2SO4, and evaporated under reduced pressure to yield a reddish solid (yield: 95%).
The characterization data agree with those reported for the same product obtained with different synthetic routes [37]. The reduction of 4-nitro-2,1,3-benzothiadiazole (BTDNO2) to afford the corresponding 4-amino-2,1,3-benzothiadiazole (BTDNH2) was carried out following a reported procedure [38], with slight modifications. To a solution containing 2.000 g of BTDNO2 (11.4 mmol) in 50 mL of ethanol, 9.208 g of FeSO4·7H2O (34.2 mmol), 4.878 g of ammonium chloride (91.2 mmol), 9 mL of water and 2.243 g of zinc dust (34.2 mmol) were added under vigorous stirring. The mixture was heated at 50 °C for three hours and, after cooling at room temperature, it was cleared by filtration on celite. The solid was washed with 3 × 10 mL of ethanol. The solution thus obtained was evaporated under reduced pressure. The crude product was dissolved in 40 mL of ethyl acetate, and 30 mL of a 25% aqueous solution of NH4Cl was added. The organic layer was extracted and washed with water (2 × 20 mL) and with 30 mL of a saturated aqueous solution of NaHCO3. The organic fraction was then dried over Na2SO4 and concentrated under reduced pressure. The product was precipitated with isohexane and dried in vacuo (yield: 45%). The characterization data were in agreement with the data reported for the same product prepared with different synthetic routes [28].
Elemental analyses (C, H, N, S) were carried out using an Elementar Unicube microanalyzer. Infrared (IR) spectra were registered using a Perkin-Elmer SpectrumOne spectrophotometer between 4000 and 400 cm−1 using KBr disks. Mono- and bidimensional nuclear magnetic resonance (NMR) spectra were collected employing Bruker Avance 300 and Avance 400 instruments operating respectively at 300.13 MHz and 400.13 MHz of 1H resonance. The 1H and 13C NMR spectra referred to the partially non-deuterated fraction of the solvent, itself referred to as tetramethylsilane.
The absorption spectra were collected in the range 235–700 nm employing a Perkin-Elmer Lambda 40 spectrophotometer. Photoluminescence emission (PL) spectra were registered at room temperature using a Horiba Jobin Yvon Fluorolog-3 spectrofluorometer. A continuous wave xenon arc lamp was used as the source, and the excitation wavelength was selected using a double Czerny–Turner monochromator. Suitable long pass filters were placed in front of the acquisition systems. The detector was composed of a single monochromator iHR320 and a photomultiplier tube Hamamatsu R928. Fluorescence quantum yields Φf of 5·×10−5 M solutions were calculated using 5 × 10−5 M anthracene in ethanol as standard based on Equation (1) [39], where Φf,std is the quantum yield of anthracene in ethanol (0.27), F and Fstd are, respectively, the areas under the fluorescence emission bands of the sample and the standard, A and Astd are respectively the absorbance values of sample and standard at the excitation wavelength, n is the refractive index of the solvent used for the sample and nstd is the refractive index of ethanol.
Φf = Φf,std·(F·Astd·n2)(Fstd·A·nstd2)−1
Electrochemical measurements were carried out on dry acetonitrile solutions of BTDNMe2 containing LiClO4 as a supporting electrolyte and ferrocene (Fc) as an internal reference. The instrument used was an eDAQ ET014-199 potentiostat, connected to eDAQ 1 mm glassy carbon disk working electrode, eDAQ 1.6 mm diameter Pt/Ti counter-electrode, and a Pt wire as a reference. Fc/Fc+ couple was used as the internal standard, and all of the measurements were carried out at room temperature under an argon atmosphere.
Computational calculations were carried out using the range-separated hybrid DFT functional ωB97X in combination with Alhrichs’ and Weigend’s def2-TZVP basis set [40,41,42,43]. The C-PCM conductor-like polarizable continuum model was added, considering acetonitrile as a continuous medium [44,45]. The TD-DFT approach was used to simulate the electronic transitions [46]. Gaussian 16 was employed as calculation software [47].
Synthesis of N,N-dimethyl-4-amino-2,1,3-benzothiadiazole, BTDNMe2: The N-methylation of 4-amino-2,1,3-benzothiadiazole (BTDNH2) was carried out by modifying a reported procedure [48]. An amount of 0.350 g of BTDNH2 (2.3 mmol) were dissolved in 15 mL of N,N-dimethylformamide (DMF), then 3.179 g of K2CO3 (23.0 mmol) and 1.4 mL of CH3I (23.0 mmol) were added under stirring. The mixture was heated at 75 °C for twelve hours. After cooling at room temperature, 50 mL of water were added, and the product was extracted with 2 × 80 mL of ethyl acetate. The organic fraction was washed with 100 mL of cold water, dried over Na2SO4, and evaporated under reduced pressure. The product was dissolved in 30 mL of pentane, the solution was purified by filtration, and the solvent was removed under nitrogen flow to afford a red oil (yield: 50%).
Characterization of Di N,N-Dimethyl-4-amino-2,1,3-benzothiadiazole.
Anal. calcd for C8H9N3S (179.24 g mol−1, %): C, 53.61; H, 5.06; N, 23.44; S, 17.89. Found (%): C, 53.40; H, 5.08; N, 23.35; S, 17.82. 1H NMR (CDCl3, 298 K) δ 7.49–7.40 (m, 2H, BTD), 6.57 (t, 1H, 3JHH = 5.9 Hz, BTD), 3.29 (s, 6H, Me). 13C {1H} NMR (CDCl3, 298 K) δ 156.90 BTD-Cipso, 149.29 BTD-Cipso, 144.43 BTD-Cipso, 130.73 BTD-CH, 111.13 BTD-CH, 108.85 BTD-CH, 42.59 Me. IR (KBr disk, cm−1): 1587 m, 1545 s, 1494 m (aromatic νC-N and νC-C).
Synthesis of BTDNMe2@PMMA: An amount of 0.250 g of PMMA was dissolved in 6 mL of dichloromethane under slow stirring, then a solution containing 0.010 g of BTDNMe2 in 4 mL of dichloromethane was added. The solution was transferred in a cylindrical polyethylene holder (1 cm diameter) and allowed to evaporate at room temperature. The polymeric film thus obtained was finally kept overnight under 10−2 torr vacuum to remove traces of solvent.
Characterization of BTDNMe2@PMMA.
PL (solid sample, λexcitation = 400 nm, nm): 606. PLE (solid sample, λemission = 605 nm, nm): <560.
3. Results and Discussion
The synthetic route here proposed for BTDNMe2 starts with the nitration of the 2,1,3-benzothiadiazole heterocycle, followed by reduction of the nitro group and subsequent methylation with methyl iodide, as depicted in Scheme 1. As observable from the 1H, 13C {1H} and 1H–13C HSQC NMR reported in Figure 1, the methyl groups are associated with a singlet at 3.29 ppm (13C resonance at 42.59 ppm), while the aromatic protons of the heterocycle resonate in the 7.50–6.50 range (13C resonances at 130.73, 111.13 and 108.85 ppm). The signals attributable to the three ipso-carbons can be detected, respectively, at 144.43, 149.28 and 156.90 ppm.
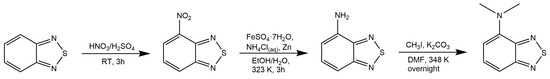
Scheme 1.
Synthesis of N,N-dimethyl-4-amino-2,1,3-benzothiadiazole, BTDNMe2.
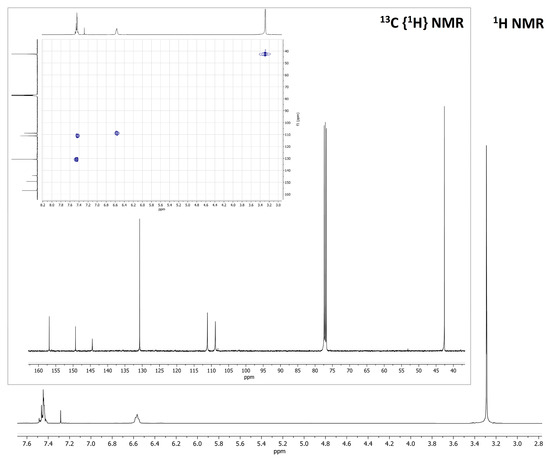
Figure 1.
1H NMR spectrum of BTDNMe2. Inset: 13C{1H} NMR and 1H-13C HSQC in CDCl3 at 298 K. CDCl3, 298 K.
The compound was isolated as a dark red oil that exhibited intriguing luminescent properties once dissolved in common organic solvents (see Figure 2). The pure oil itself did not display appreciable emissions probably because of concentration quenching. The evident solvatochromism was investigated considering four solvents characterized by different dielectric constants ε (n-hexane, dichloromethane, acetone, and acetonitrile). The absorption and emission spectra are shown in Figure 2. The selected properties of the solvents, including the orientation polarizability Δf (see Equation (2)), are summarized in Table 1. The table also reports the absorption and emission maxima of BTDNMe2, Stokes shifts ῦA-ῦF and Φf values, calculated accordingly to Equation (1).
Δf = (ε − 1)(2ε + 1)−1 − (n2 − 1)(2n2 + 1)−1
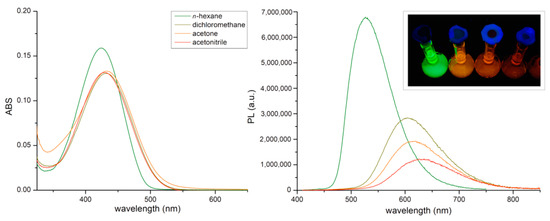
Figure 2.
Absorption (left) and emission (right) spectra of 5 × 10−5 M solutions of BTDNMe2 in different solvents recorded at room temperature. Inset: picture of the solutions under UV light (λexcitation = 365 nm).

Table 1.
Fluorescence data of BTDNMe2 in different solvents.
As presented in Figure 2 and Table 1, the solvents characterized by higher ε values determine a bathochromic shift of the emission maxima in solution together with an increase in the Stokes shift, which varies from 4559 cm−1 in hexane to 7448 cm−1 in acetonitrile (see Table 1). The greatest variations occur on changing the solvent from hexane to dichloromethane, with a shift of the emission maximum from 526 to 604 nm and a consequent increase of the Stokes shift from 4559 cm−1 to 6613 cm−1. The CIE 1931 chromaticity coordinates are reported in the diagram in Figure 3, where the change of emission is observable from yellowish-green to reddish-orange on increasing the ε value. The colour purity of the emission of BTDNMe2 in hexane is 0.79, while it is almost unitary for the other solvents.
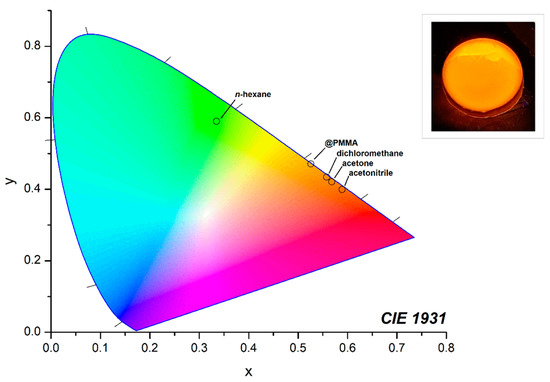
Figure 3.
CIE 1931 chromaticity diagram of BTDNMe2 in different solvents and in PMMA (n-hexane: x = 0.335, y = 0.590; dichloromethane: x = 0.558, y = 0.434; acetone: x = 0.561, y = 0.421; acetonitrile: x = 0.589, y = 0.399; @PMMA: x = 0.526, y = 0.471). Inset: BTDNMe2@PMMA excited at 365 nm.
The increase of the dielectric constant also causes a decrease in the fluorescence quantum yield, from 52% (hexane) to 16% (acetonitrile), probably attributable to the relative increase of non-radiative decay because of the red-shift of the emission.
As observable in Figure 4, the Stokes shift ῦA-ῦF increases roughly linearly with the orientation polarizability Δf (Pearson’s coefficient R = 0.99), accordingly to the Lippert–Mataga equation (Equation (3)) [50,51]. h is Planck’s constant, c is the speed of light in a vacuum, as is the radius of the cavity in which the molecule resides, and μe and μg are the dipole moments of the excited and ground state, respectively. The plot in Figure 4 confirms that the solvatochromic effect is related to specific solute–solvent interactions that involve the polarization [52].
ῦA − ῦF = 2·h−1c−1·(μe − μg)2·as−3·Δf + constant
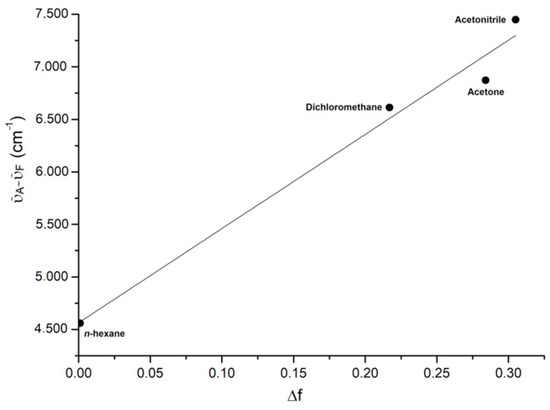
Figure 4.
Lippert-Mataga plot.
The radius obtained from the C-PCM/DFT optimization of the structure of BTDNMe2 is 3.92 Å. Based on Equation (3), the increase of dipole moment from the ground to the excited state is about 7 D.
The luminescent properties of BTDNMe2 were maintained after encapsulation in the PMMA matrix. The emission falls in the orange region of the CIE diagram with unitary colour purity, as observable in the CIE 1931 chromaticity diagram and the picture reported as an inset in Figure 3.
The photoluminescent properties were justified by means of electrochemical measurements and DFT calculations. As observable from the square wave voltammetry reported in Figure 5, the HOMO–LUMO gap can be estimated at around 2.5 eV, considering the irreversible oxidation and reduction processes. Such an outcome is in agreement with the onset of the absorption spectrum using acetonitrile as solvent. The TD-DFT calculations confirm that the lowest energy transition occurs between HOMO and LUMO, which are the π and π* frontier molecular orbitals mostly localized on the benzothiadiazole skeleton, with a contribution from both the molecular orbitals of the N,N-dimethylamino moiety (Figure 5).
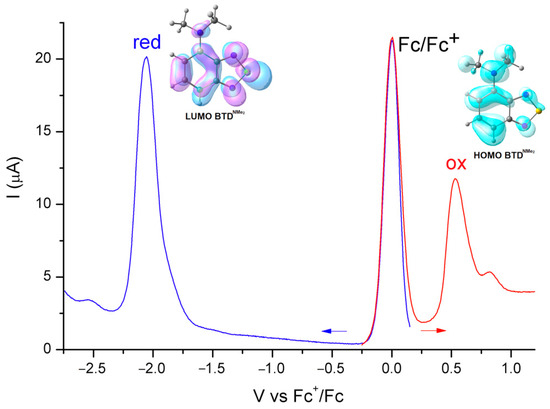
Figure 5.
Square wave voltammetry of BTDNMe2 (CH3CN/LiClO4, ferrocene as internal reference, blue line: reduction, red line: oxidation) and frontier molecular orbitals (surface isovalue = 0.03 a.u.).
4. Conclusions
N,N-Dimethyl-4-amino-2,1,3-benzothiadiazole (BTDNMe2) was prepared from 2,1,3-benzothiadiazole in a three-step synthetic path that involved nitration, subsequent reduction and methylation. The compound was fully characterized by means of nuclear magnetic resonance (NMR) and infrared spectroscopy. The compound was revealed to be highly fluorescent and characterized by a noticeable solvatochromism. The emission features rationalized based on electrochemical measurements and DFT calculations were maintained once embedded in the poly(methyl methacrylate). The photoluminescence properties exhibited by the BTDNMe2 make it a suitable candidate for advanced technology applications, and further functionalizations are currently under investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecsoc-25-11658/s1.
Author Contributions
Conceptualization, V.F. and M.B.; methodology, M.B.; software, V.F. and M.B.; validation, M.B.; formal analysis, V.F. and M.B.; investigation, V.F., M.G. and M.B.; resources, M.B.; data curation, V.F. and M.B.; writing—original draft preparation, V.F. and M.B.; writing—review and editing, V.F. and M.B.; visualization, M.B.; supervision, V.F. and M.B.; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Università Ca’ Foscari Venezia, Bando Spin 2018, D. R. 1065/2018 prot. 67416.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
Università Ca’ Foscari Venezia is gratefully acknowledged for financial support (Bando Spin 2018, D. R. 1065/2018 prot. 67416). We acknowledge CINECA (COLUMN and COLUMN21 projects) for the availability of high-performance computing resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neto, B.A.D.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-benzothiadiazole and derivatives: Synthesis, properties, reactions, and applications in light technology of small molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Ogienko, D.S.; Bashirov, D.A.; Konchenkoa, S.N. Luminescent complexes of 2,1,3-benzothiadiazole derivatives. Russ. Chem. Bull. 2019, 68, 651–661. [Google Scholar] [CrossRef]
- Langis-Barsetti, S.; Maris, T.; Wuest, J.D. Molecular organization of 2,1,3-benzothiadiazoles in the solid state. J. Org. Chem. 2017, 82, 5034–5045. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Takahashi, T.; Sugawara, R.; Lo, C.-T.; Mori, H. Benzothiadiazole-based donor–acceptor nanoparticles with solvatochromic and thermoresponsive properties. React. Funct. Polym. 2018, 131, 350–360. [Google Scholar] [CrossRef]
- Benevides, T.O.; Regis, E.; Nicoleti, C.S.; Bechtold, I.H.; Vieira, A.A. Phase-dependent photoluminescence of non-symmetric 2,1,3-benzothiadiazole liquid crystals. Dye. Pigment. 2019, 163, 300–307. [Google Scholar] [CrossRef]
- Zhao, X.; Chaudhry, S.T.; Mei, J. Heterocyclic building blocks for organic semiconductors. In Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 121, pp. 133–171. [Google Scholar] [CrossRef]
- Volz, D.; Wallesch, M.; Fléchon, C.; Danz, M.; Verma, A.; Navarro, J.M.; Zink, D.M.; Bräse, S.; Baumann, T. From iridium and platinum to copper and carbon: New avenues for more sustainability in organic light-emitting diodes. Green Chem. 2017, 17, 1988–2011. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Qu, J.; Qian, P.-C.; Wong, W.-Y. Recent progress of electronic materials based on 2,1,3-benzothiadiazole and its derivatives: Synthesis and their application in organic light-emitting diodes. Sci. China Chem. 2021, 64, 341–357. [Google Scholar] [CrossRef]
- Pazini, A.; Maqueira, L.; Stieler, R.; Aucélio, R.Q.; Limberger, J. Synthesis, characterization and photophysical properties of luminescent non-symmetric 4-pyridyl benzothiadiazole derivatives. J. Mol. Struct. 2017, 1131, 181–189. [Google Scholar] [CrossRef]
- Bardi, B.; Dall’Agnese, C.; Moineau-Chane Ching, K.I.; Painelli, A.; Terenziani, F. Spectroscopic investigation and theoretical modeling of benzothiadiazole-based charge-transfer chromophores: From solution to nanoaggregates. J. Phys. Chem. C 2017, 121, 17466–17478. [Google Scholar] [CrossRef]
- Pazini, A.; Maqueira, L.; Avila, H.C.; Valente, F.M.; Aderne, R.E.; Back, D.; Aucélio, R.Q.; Cremona, M.; Limberger, J. Phenoxy-benzothiadiazole dyes: Synthesis, photophysical properties and preliminary application in OLEDs. Tetrahedron Lett. 2018, 59, 2994–2999. [Google Scholar] [CrossRef]
- Paczkowski, I.M.; Coelho, F.L.; Campo, L.F. 2,1,3-Benzothiadiazole dyes conjugated with benzothiazole and benzoxazole: Synthesis, solvatochromism and solid-state properties. J. Mol. Liq. 2020, 319, 114277. [Google Scholar] [CrossRef]
- Paisley, N.R.; Tonge, C.M.; Mayder, D.M.; Thompson, K.A.; Hudson, Z.M. Tunable benzothiadiazole-based donor–acceptor materials for two-photon excited fluorescence. Mater. Chem. Front. 2020, 4, 555–566. [Google Scholar] [CrossRef]
- Gao, S.; Balan, B.; Yoosaf, K.; Monti, F.; Bandini, E.; Barbieri, A.; Armaroli, N. Highly efficient luminescent solar concentrators based on benzoheterodiazole dyes with large stokes shifts. Chem. Eur. J. 2020, 26, 11013–11023. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic sensitizers from D–π–A to D–A–π–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef]
- Holliday, S.; Li, Y.; Luscombe, C.K. Recent advances in high performance donor-acceptor polymers for organic photovoltaics. Prog. Polym. Sci. 2017, 70, 34–51. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, J.; Peng, F.; Peng, S.; Liao, J.; Zhao, H.; Li, L.; Zeng, X.; Yu, H. Novel dual acceptor (D–D′–A′–π–A) dye-sensitized solar cells based on the triarylamine structure and benzothiadiazole double electron withdrawing unit. New J. Chem. 2021, 45, 4443–4452. [Google Scholar] [CrossRef]
- Wang, C.; Liu, F.; Chen, Q.-M.; Xiao, C.-Y.; Wu, Y.-G.; Li, W.-W. Benzothiadiazole-based conjugated polymers for organic solar cells. Chin. J. Polym. Sci. 2021, 39, 525–536. [Google Scholar] [CrossRef]
- Raitz, I.; de Souza Filho, R.Y.; de Andrade, L.P.; Correa, J.R.; Neto, B.A.D.; Pilli, R.A. Preferential mitochondrial localization of a goniothalamin fluorescent derivative. ACS Omega 2017, 2, 3774–3784. [Google Scholar] [CrossRef] [Green Version]
- Appelqvist, H.; Stranius, K.; Börjesson, K.; Nilsson, K.P.R.; Dyrager, C. Specific imaging of intracellular lipid droplets using a benzothiadiazole derivative with solvatochromic properties. Bioconjug. Chem. 2017, 28, 1363–1370. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.S.; Corrêa, J.R.; Carvalho, P.H.; Zanotto, G.M.; Matiello, G.I.; Guido, B.C.; Gatto, C.C.; Ebeling, G.; Gonçalves, P.F.B.; Dupont, J.; et al. Appending ionic liquids to fluorescent benzothiadiazole derivatives: Light up and selective lysosome staining. Sens. Actuators B Chem. 2020, 321, 128530. [Google Scholar] [CrossRef]
- Uchiyama, S.; Tsuji, T.; Ikado, K.; Yoshida, A.; Kawamoto, K.; Hayashic, T.; Inadac, N. A cationic fluorescent polymeric thermometer for the ratiometric sensing of intracellular temperature. Analyst 2015, 140, 4498–4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, S.; Kimura, K.; Gota, C.; Okabe, K.; Kawamoto, K.; Inada, N.; Yoshihara, T.; Tobita, S. Environment-sensitive fluorophores with benzothiadiazole and benzoselenadiazole structures as candidate components of a fluorescent polymeric thermometer. Chem. Eur. J. 2012, 18, 9552–9563. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Byun, J.; Huang, W.; Ayed, C.; Wang, L.; Zhang, K.A.I. Poly(benzothiadiazoles) and their derivatives as heterogeneous photocatalysts for visible-light-driven chemical transformations. ACS Catal. 2018, 8, 4735–4750. [Google Scholar] [CrossRef]
- Wang, G.-B.; Li, S.; Yan, C.-X.; Lin, Q.-Q.; Zhu, F.-C.; Geng, Y.; Dong, Y.-B. A benzothiadiazole-based covalent organic framework for highly efficient visible-light driven hydrogen evolution. Chem. Commun. 2020, 56, 12612–12615. [Google Scholar] [CrossRef] [PubMed]
- Broncová, G.; Shishkanova, T.V.; Dendisová, M.; Člupek, M.; Kubáč, D.; Matějka, P. Poly(4-amino-2,1,3-benzothiadiazole) films: Preparation, characterization and applications. Chem. Pap. 2017, 71, 359–366. [Google Scholar] [CrossRef]
- Munakata, M.; He, H.; Kuroda-Sowa, T.; Maekawa, M.; Suenaga, Y. Dicopper complexes derived from 4-amino-2,1,3-benzothiadiazole with versatile co-ordination number and geometry. J. Chem. Soc. Dalton Trans. 1998, 1499–1502. [Google Scholar] [CrossRef]
- Bashirov, D.A.; Sukhikh, T.S.; Kuratieva, N.V.; Chulanova, E.A.; Yushina, I.V.; Gritsan, N.P.; Konchenko, S.N.; Zibarev, A.V. Novel applications of functionalized 2,1,3-benzothiadiazoles for coordination chemistry and crystal engineering. RSC Adv. 2014, 4, 28309–28316. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Ogienko, D.S.; Bashirov, D.A.; Kuratieva, N.V.; Komarov, V.Y.; Rakhmanova, M.I.; Konchenko, S.N. New red-luminescent cadmium coordination polymers with 4-amino-2,1,3-benzothiadiazole. J. Coord. Chem. 2016, 69, 3284–3293. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Bashirov, D.A.; Ogienko, D.S.; Kuratieva, N.V.; Sherin, P.S.; Rakhmanova, M.I.; Chulanova, E.A.; Gritsan, N.P.; Konchenko, S.N.; Zibarev, A.V. Novel luminescent β-ketoimine derivative of 2,1,3-benzothiadiazole: Synthesis, complexation with Zn(ii) and photophysical properties in comparison with related compounds. RSC Adv. 2016, 6, 43901–43910. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Komarov, V.Y.; Konchenko, S.N.; Benassi, E. The hows and whys of peculiar coordination of 4-amino-2,1,3-benzothiadiazole. Polyhedron 2018, 139, 33–43. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Bashirov, D.A.; Shuvaev, S.; Komarov, V.Y.; Kuratieva, N.V.; Konchenko, S.N.; Benassi, E. Noncovalent interactions and photophysical properties of new Ag(I) complexes with 4-amino-2,1,3-benzothiadiazole. Polyhedron 2018, 141, 77–86. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Khisamov, R.M.; Bashirov, D.A.; Komarov, V.Y.; Molokeev, M.S.; Ryadun, A.A.; Benassi, E.; Konchenko, S.N. Tuning of the coordination and emission properties of 4-amino-2,1,3-benzothiadiazole by introduction of diphenylphosphine group. Cryst. Growth Des. 2020, 20, 5796–5807. [Google Scholar] [CrossRef]
- Slavachevskaya, N.M.; Belen’kaya, I.A.; Tsepova, N.S.; Levocheskaya, E.I.; Krasil’nikov, I.I. Synthesis of certain quaternary derivatives in the aminophenol and benzo-2,1,3-thiadiazole series as potential radiation-protecting materials. Pharm. Chem. J. 1976, 10, 327–331. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Komin, A.P.; Carmack, M. The chemistry of 1,2,5-thiadiazoles, IV. Benzo [1,2-c:3,4-c′:5,6-c″]tris [1,2,5]thiadiazole. J. Heterocycl. Chem. 1975, 12, 829–833. [Google Scholar] [CrossRef]
- Da Silva Miranda, F.; Signori, A.M.; Vicente, J.; de Souza, B.; Priebe, J.P.; Szpoganicz, B.; Sanches Gonçalves, N.; Neves, A. Synthesis of substituted dipyrido[3,2-a:2′,3′-c]phenazines and a new heterocyclic dipyrido[3,2-f:2′,3′-h]quinoxalino[2,3-b]quinoxaline. Tetrahedron 2008, 64, 5410–5415. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Prashad, M.; Repič, O.; Blacklock, T.J. A practical and chemoselective reduction of nitroarenes to anilines using activated iron. Adv. Synth. Catal. 2005, 347, 217–219. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Lavabre, D. Are fluorescence quantum yields so tricky to measure? a demonstration using familiar stationery products. J. Chem. Educ. 1999, 76, 1260–1264. [Google Scholar] [CrossRef]
- Gerber, I.C.; Ángyán, J.G. Hybrid functional with separated range. Chem. Phys. Lett. 2005, 415, 100–105. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Minenkov, Y.; Singstad, Å.; Occhipinti, G.; Jensen, V.R. The accuracy of DFT-optimized geometries of functional transition metal compounds: A validation study of catalysts for olefin metathesis and other reactions in the homogeneous phase. Dalton Trans. 2012, 41, 5526–5541. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the CPCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solven-model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Ullrich, C.A. Time-Dependent Density Functional Theory; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Reddy, M.D.; Fronczek, F.R.; Watkins, E.B. Rh-catalyzed, regioselective, C–H bond functionalization: Access to quinoline-branched amines and dimers. Org. Lett. 2016, 18, 5620–5623. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence: Principles and Applications; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent effects upon fluorescence spectra and the dipole moments of excited molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Singapore; Japan, 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).