Abstract
Fabrication and characterization of CH3NH3PbI3 perovskite solar cell with the addition of copper (Cu2+), and potassium (K) or guanidinium (GA) was performed. The additive effects on the photovoltaic properties, morphologies, and crystalline structures were investigated by the experimental results, electronic structures, and thermodynamic stabilities. The stability and conversion efficiency of the perovskite solar cells was improved by incorporating Cu2+ at the lead site, and K or GA at the organic cation, CH₃NH₃, at A-site in cubic crystal. The simultaneous addition of Cu2+ and K to the perovskite crystal suppressed the crystal decomposition while inhibiting desorption of MA, improving the stability of the performance.
1. Introduction
The semiconductor lead halide perovskite (LHP) attracted attention as the active layer of LEDs in the 1990s, and in the 2000s it was rapidly developed as the thin-film absorber layer of solar cells, and has been actively researched worldwide [1]. There is also a growing number of computational simulation works [2,3,4,5,6]. Although perovskite solar cells (PSC) have the advantage of being inexpensive to produce, they have serious problems of low durability and environmental pollution by Pb [7]. The durability of PSC is caused mainly by the decomposition of the perovskite crystals due to methylammonium (MA) desorption. To solve these problems, attempts to improve the interface of perovskites and to introduce additives into the perovskite layer to improve the electronic properties have been studied [8,9,10,11]. Previous studies have reported that substitution of guanidinium (GA), which is larger than MA, can inhibit MA desorption, resulting in improved performance and long-term stability [12,13,14,15]. In addition, it has been reported that the introduction of potassium (K) improves the electron transport layer (ETL) and perovskite interface state [16,17,18,19]. It has also been reported that the addition of environmentally benign transition metals other than Pb reduces toxicity and changes the electronic state to improve the performance of PSC [20,21,22,23]. Among them, the environmentally friendly transition metal Cu2+ has been considered as a candidate for Pb replacement, but there are few reported cases [24,25,26,27,28].
The aim of this work is to fabricate and characterize devices doped with Cu2+, GA, and K. The photovoltaic properties, morphologies, and crystal structure were investigated by substitution of Cu2+, GA, and K. The stability of the performance was measured. In addition, first-principles calculations were compared with experimental results.
2. Experimental Procedures
The preparation of perovskite solar cells is shown in Figure 1 [29,30,31,32]. The FTO (F-doped Tin Oxide) substrates were ultrasonically cleaned with acetone and methanol and dried under nitrogen gas. TiO2 precursor solution (0.15, 0.30 M) was prepared by adding 1-butanol (1 mL) to titanium diisopropoxide bis(acetylacetonate) (Sigma–Aldrich, St. Louis, MO, USA, 0.055, 0.11 mL). The 0.15 M TiO2 precursor solution was spin-coated onto the FTO substrate at 3000 rpm for 30 s, and then the coated substrate was annealed at 125 °C for 5 min. 0.30 M TiO2 precursor solution was spin-coated onto the TiO2 layer at 3000 rpm for 30 s, and then the resulting substrate was annealed at 125 °C for 5 min. This process of forming the 0.30 M precursor layer was carried out twice. The FTO substrate was then baked at 550 °C for 30 min to form a compact TiO2 layer. To form the mesoporous TiO2 layer, TiO2 paste was prepared by mixing TiO2 powder (Aerosil, Tokyo, Japan, P-25, 200 mg) and poly(ethylene glycol) (Wako Pure Chemical Corporation, Osaka, Japan, PEG #20000 20 mg) with ultrapure water (1 mL). To this solution, acetylacetone (Wako Pure Chemical Corporation, Osaka, Japan, 20 µL) and surfactant (Sigma–Aldrich, St. Louis, MO, USA, Triton X-100, 10 µL) were added, mixed for 30 min, and then allowed to stand for 24 h to remove bubbles in the solution. The TiO2 paste was then spin-coated on the compact TiO2 layer at 5000 rpm for 30 s. The resulting cell was heated at 125 °C for 5 min and then annealed at 550 °C for 30 min to form a mesoporous TiO2 layer. To prepare the perovskite compound, a mixture of CH3NH3I (2.4 M, Tokyo Chemical Industry, Tokyo, Japan), PbCl2 (0.8 M, Sigma–Aldrich, St. Louis, MO, USA) in DMF (Nacalai Tesque, Kyoto, Japan, 0.5 mL) solution was prepared for the standard cell. Then, these perovskite solutions were spin-coated on TiO2 at 2000 rpm for 60 s with air blow. This process was performed three times: a solution of DPPS (Osaka Gas Chemical, OGSOL SI-30-15, Osaka, Japan, 10 mg) was prepared in chlorobenzene (0.5 mL) and dropped onto the perovskite layer during the last 15 s of the third spin coating of the perovskite precursor solution [33,34,35,36,37]. Subsequently, annealing was performed at 200 °C in air. Spiro-OMeTAD (Sigma–Aldrich, St. Louis, MO, USA, 36.1 mg) was dissolved in chlorobenzene (Wako Pure Chemical corporation, 0.5 mL). Lithium bis(trifluoromethylsulfonyl)imide (Li-TFSI, Tokyo Chemical Industry, Tokyo, Japan, 260 mg) and FK209 (Sigma–Aldrich, St. Louis, MO, USA, 188 mg) were each added to acetonitrile (Sigma–Aldrich, St. Louis, MO, USA, 0.5 mL). Immediately before membrane formation, 4-tertbutylpyridine (Sigma–Aldrich, St. Louis, MO, USA, 18 μL), the prepared solution of Li-TFSI (10 μL), and the solution of FK209 (4 μL) were mixed at 70 °C for 30 min. The spiro-OMeTAD solution was then spin-coated on the perovskite layer at 4000 rpm for 30 s. All procedures were performed in air. Finally, a gold (Au) electrode was deposited to serve as the top electrode. The layer structure of the prepared solar cell is FTO/TiO2/perovskite/spiro-OMeTAD/Au. The prepared perovskite solar cells were stored at a temperature of 22 °C and humidity below 30%.
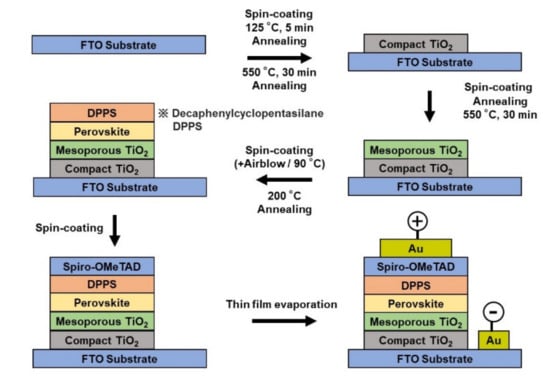
Figure 1.
Schematics the processes for the PSC architectures.
3. Results and Discussion
3.1. First-Principles Calculation
The electron distribution diagrams and density of states (DOS) of MAPbI3 and Cu2+-doped MAPbCuI3 were calculated by first-principles calculations [38,39,40,41,42,43,44]. MAPbCuI3 increase in DOS due to the overlap of Cu2+ 3d and I 5p orbitals near the HOMO. This indicates charge transfer from I 5p orbitals through Cu2+ 3d orbitals. From the results, it is predicted that the short-circuit current density (JSC) increases due to the easier hole migration. The calculation of the band gap (Eg) suggested that the simultaneous addition of Cu2+ and K would expand the Eg related to the open circuit voltage (VOC). Eg is the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO).
The cubic and crystal models doped with Cu2+ and GA are shown in Figure 2. From the energy calculation, the GA-doped system had increase of energy, yielding unstable state, as compared to the standard system. These results suggest that the stability of the performance of the perovskite crystal is improved by the simultaneous addition of Cu2+ and K. In the other case, the perovskite crystal with addition of GA might occur the crystal decomposition with the distortion of MA.
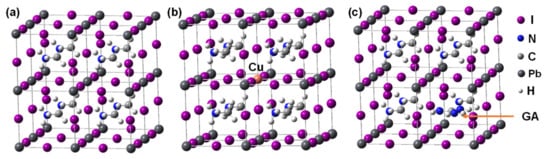
Figure 2.
Crystal structures of cubic (a) MAPbI3, (b) MAPb0.963Cu0.037I3, and (c) MA0.875GA0.125PbI3.
3.2. Device Characterization
Immediately after fabrication, the current–voltage characteristic (J–V) curve and the external quantum efficiency (EQE) spectrum were measured. The performance of the Cu2+ 2% + K 2% doped device was inferior to that of the standard device. The surface observation of SEM and EDX analysis showed uniform morphologies and monodispersed crystal grain in the perovskite crystals containing a slight K. The GA-doped perovskite crystal exhibited a tetragonal crystal system, consistent with calculated predictions and X-ray diffraction pattern.
The stability of conversion efficiency of the devices was investigated. The performance of the devices doped with Cu2+ and K simultaneously maintained to be about 10.8% after 28 days, while the performance of the standard devices was reduced by about 8.5%. The optical micrographs showed grain growth with increase of size. The XRD pattern indicate the lattice constant of the crystals was decreased by doping Cu2+ and K.
The schematic model of atomic diffusion in the Cu2+, K-added perovskite crystals was discussed. The lattice constant of the perovskite crystal was considered to have decreased by the substitution of K, which was not added immediately after the device fabrication, to the desorption position of MA with the passage of time. The simultaneous addition of Cu2+ and K to the perovskite crystal decreased the lattice constant, yielding wide bandgap related to VOC, as compared to those in the MAPbI3 perovskite crystal.
4. Conclusions
The effects of the co-addition of CuCl2, KI, and GAI to MAPbI3 on the photovoltaic properties, microstructures, and crystal structure were investigated. The calculation of the Eg suggested that the simultaneous addition of Cu2+ and K would increase expansion of the Eg related to the VOC and to improve performance. Furthermore, it was found that the devices with 2% Cu2+ and 2% K-doping had the best stability and performance. The addition of K in the perovskite layer promoted the uniform crystal growth with an increase of grain size while inhibiting crystal decomposition, yielding greater stability of performance.
Author Contributions
Conceptualization, A.E., A.S. and T.O.; methodology, A.E., A.S. and T.O.; formal analysis, A.E., A.S. and T.O.; investigation, A.E.; resources, A.E., A.S., T.O., M.O., S.F., T.T. and T.H.; data curation, A.E., A.S. and T.O.; writing—original draft preparation, A.E., A.S. and T.O.; writing—review and editing, A.S. and T.O.; visualization, A.E., A.S. and T.O.; project administration, A.S. and T.O.; funding acquisition, A.S. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Japan Society for the Promotion of Science as a Grants-in-Aid for Scientific Research (C) JP21K05261.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umari, P.; Mosconi, E.; De Angelis, F. Relativistic GW calculations on CH3NH3PbI3 and CH3NH3SnI3 perovskites for solar cell applications. Sci. Rep. 2014, 4, 4467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brivio, F.; Frost, J.M.; Skelton, J.; Jackson, A.; Weber, O.; Weller, M.; Goñi, A.R.; Leguy, A.M.A.; Barnes, P.; Walsh, A. Lattice dynamics and vibrational spectra of the orthorhombic, tetragonal, and cubic phases of methylammonium lead iodide. Phys. Rev. B 2015, 92, 14. [Google Scholar] [CrossRef] [Green Version]
- Bu, T.; Li, J.; Li, H.; Tian, C.; Su, J.; Tong, G.; Ono, L.K.; Wang, C.; Lin, Z.; Chai, N.; et al. Lead halide–templated crystallization of methylamine-free perovskite for efficient photovoltaic modules. Science 2021, 372, 1327–1332. [Google Scholar] [CrossRef]
- Ashari-Astani, N.; Meloni, S.; Salavati, A.H.; Palermo, G.; Grätzel, M.; Rothlisberger, U. Computational characterization of the dependence of halide perovskite effective masses on chemical composition and structure. J. Phys. Chem. C 2017, 121, 23886–23895. [Google Scholar] [CrossRef]
- Qiao, L.; Fang, W.-H.; Long, R.; Prezhdo, O.V. Photoinduced dynamics of charge carriers in metal halide perovskites from an atomistic perspective. J. Phys. Chem. Lett. 2020, 11, 7066–7082. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Zakeeruddin, S.M.; Grätzel, M.; Fan, Z. Large-grain tin-rich perovskite films for efficient solar cells via metal alloying technique. Adv. Mater. 2018, 30, 11. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, K.; Guo, D.Y.; Wang, S.L.; Li, P.G. Cations substitution tuning phase stability in hybrid perovskite single crystals by strain relaxation. RSC Adv. 2018, 8, 2900–2905. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Teng, P.; Xu, W.; Zheng, G.; Lin, W.; Yin, J.; Kobera, L.; Abbrent, S.; Li, X.; Steele, J.A.; et al. Manipulating crystallization dynamics through chelating molecules for bright perovskite emitters. Nat. Commun. 2021, 12, 4831. [Google Scholar] [CrossRef]
- Wang, Y.; Mahmoudi, T.; Hahn, Y. Highly stable and efficient perovskite solar cells based on FAMA-perovskite-Cu:NiO composites with 20.7% efficiency and 80.5% fill factor. Adv. Energy Mater. 2020, 10, 27. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, Z.; Yu, F.; Yang, D.; Liu, S.F. Alkali metal doping for improved CH3NH3PbI3 perovskite solar cells. Adv. Sci. 2018, 5, 1700131. [Google Scholar] [CrossRef] [Green Version]
- Jodlowski, A.Z.D.; Carmona, C.R.; Grancini, G.; Salado, M.; Ralaiarisoa, M.; Ahmad, S.; Koch, N.; Camacho, L.; de Miguel, G.; Nazeeruddin, M.K. Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells. Nat. Energy 2017, 2, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Xiong, J.; Li, J.; Daoud, W.A. Guanidinium induced phase separated perovskite layer for efficient and highly stable solar cells. J. Mater. Chem. A 2019, 7, 9486–9496. [Google Scholar] [CrossRef]
- Kishimoto, T.; Suzuki, A.; Ueoka, N.; Oku, T. Effects of guanidinium addition to CH3NH3PbI3−xClx perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 2019, 127, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Ji, S.; Grandhi, G.K.; Bin Cho, H.; Bin Im, W.; Park, J. Multimodal digital X-ray scanners with synchronous mapping of tactile pressure distributions using perovskites. Adv. Mater. 2021, 33, 2008539. [Google Scholar] [CrossRef]
- Tang, Z.; Bessho, T.; Awai, F.; Kinoshita, T.; Maitani, M.; Jono, R.; Murakami, T.N.; Wang, H.; Kubo, T.; Uchida, S.; et al. Hysteresis-free perovskite solar cells made of potassium-doped organometal halide perovskite. Sci. Rep. 2017, 7, 12183. [Google Scholar] [CrossRef]
- Alanazi, T.I.; Game, O.S.; Smith, J.A.; Kilbride, R.C.; Greenland, C.; Jayaprakash, R.; Georgiou, K.; Terrill, N.J.; Lidzey, D.G. Potassium iodide reduces the stability of triple-cation perovskite solar cells. RSC Adv. 2020, 10, 40341–40350. [Google Scholar] [CrossRef]
- Kandori, S.; Oku, T.; Nishi, K.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and characterization of potassium- and formamidinium-added perovskite solar cells. J. Ceram. Soc. Jpn. 2020, 128, 805–811. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, J.; Walter, H.; Kazi, A.; Wang, D.; Wangila, G.; Mortazavi, M.; Yan, C.; Jiang, Q. The doping of alkali metal for halide perovskites. ES Mater. Manuf. 2020, 7, 25–33. [Google Scholar] [CrossRef]
- Klug, M.T.; Osherov, A.; Haghighirad, A.A.; Stranks, S.D.; Brown, P.R.; Bai, S.; Wang, J.T.-W.; Dang, X.; Bulović, V.; Snaith, H.J.; et al. Tailoring metal halide perovskites through metal substitution: Influence on photovoltaic and material properties. Energy Environ. Sci. 2016, 10, 236–246. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Tewari, N.; Ng, M.; Geng, P.; Chen, D.; Ko, P.K.; Qammar, M.; Guo, L.; Halpert, J.E. Progress in copper metal halides for optoelectronic applications. Mater. Chem. Front. 2021, 5, 4796–4820. [Google Scholar] [CrossRef]
- Karthick, S.; Hawashin, H.; Parou, N.; Vedraine, S.; Velumani, S.; Boucle, J. Copper and bismuth incorporated mixed cation perovskite solar cells by one-step solution process. Solar Energy. 2021, 218, 226–236. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.K.; Zhuo, M.P.; Hu, Y.; Hu, K.H.; Ye, Q.Q.; Jain, S.M.; Yang, Y.G.; Gao, X.Y.; Liao, L.S. Pb-Sn-Cu ternary organometallic halide perovskite solar cells. Adv. Mater. 2018, 30, e1800258. [Google Scholar] [CrossRef]
- Elseman, A.M.; Shalan, A.E.; Sajid, S.; Rashad, M.M.; Hassan, A.M.; Li, M. Copper-substituted lead perovskite materials constructed with different halides for working (CH3NH3)2CuX4-based perovskite solar cells from experimental and theoretical view. ACS Appl. Mater. Interfaces 2018, 10, 11699–11707. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Effects of doping with Na, K, Rb, and formamidinium cations on (CH3NH3)0.99Rb0.01Pb0.99Cu0.01I3−x(Cl,Br)x perovskite photovoltaic cells. AIP Adv. 2020, 10, 125023. [Google Scholar] [CrossRef]
- Ge, X.; Qu, X.; He, L.; Sun, Y.; Guan, X.; Pang, Z.; Wang, C.; Yang, L.; Wang, F.; Rosei, F. 3D low toxicity Cu–Pb binary perovskite films and their photoluminescent/photovoltaic performance. J. Mater. Chem. A 2019, 7, 27225–27235. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T. Effects of co-addition of sodium chloride and copper(ii) bromide to mixed-cation mixed-halide perovskite photovoltaic devices. ACS Appl. Energy Mater. 2020, 3, 7272–7283. [Google Scholar] [CrossRef]
- Wang, K.-L.; Wang, R.; Wang, Z.-K.; Li, M.; Zhang, Y.; Ma, H.; Liao, L.-S.; Yang, Y. Tailored phase transformation of CsPbI2Br films by copper(ii) bromide for high-performance all-inorganic perovskite solar cells. Nano Lett. 2019, 19, 5176–5184. [Google Scholar] [CrossRef]
- Oku, T.; Zushi, M.; Imanishi, Y.; Suzuki, A.; Suzuki, K. Microstructures and photovoltaic properties of perovskite-type CH3NH3PbI3 compounds. Appl. Phys. Express 2014, 7, 121601. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y.; Ueoka, N. Highly (100)-oriented CH3NH3PbI3(Cl) perovskite solar cells prepared with NH4Cl using an air blow method. RSC Adv. 2018, 8, 10389–10395. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Oe, M.; Oku, T. Fabrication and characterization of Ni-, Co-, and Rb-incorporated CH3NH3PbI3 perovskite solar cells. J. Electron. Mater. 2021, 50, 1980–1995. [Google Scholar] [CrossRef]
- Oku, T. Crystal structures of perovskite halide compounds used for solar cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Taguchi, M.; Suzuki, A.; Oku, T.; Ueoka, N.; Minami, S.; Okita, M. Effects of annealing temperature on decaphenylcyclopentasilane-inserted CH3NH3PbI3 perovskite solar cells. Chem. Phys. Lett. 2019, 737, 136822. [Google Scholar] [CrossRef]
- Oku, T.; Kandori, S.; Taguchi, M.; Suzuki, A.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Polysilane-inserted methylammonium lead iodide perovskite solar cells doped with formamidinium and potassium. Energies 2020, 13, 4776. [Google Scholar] [CrossRef]
- Suzuki, A.; Taguchi, M.; Oku, T.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Additive effects of methyl ammonium bromide or formamidinium bromide in methylammonium lead iodide perovskite solar cells using decaphenylcyclopentasilane. J. Mater. Sci. Mater. Electron. 2021, 32, 26449–26464. [Google Scholar] [CrossRef]
- Oku, T.; Taguchi, M.; Suzuki, A.; Kitagawa, K.; Asakawa, Y.; Yoshida, S.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Effects of polysilane addition to chlorobenzene and high temperature annealing on CH3NH3PbI3 perovskite photovoltaic devices. Coatings 2021, 11, 665. [Google Scholar] [CrossRef]
- Suzuki, A.; Kitagawa, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T. Additive effects of copper and alkali metal halides into methylammonium lead iodide perovskite solar cells. Electron. Mater. Lett. 2021, 18, 176–186. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Additive effects of alkali metals on Cu-modified CH3NH3PbI3−δClδ photovoltaic devices. RSC Adv. 2019, 9, 24231–24240. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Oku, T. Electronic structures and magnetic properties of transition metal doped CsPbI3 perovskite compounds by first-principles calculation. Phys. Solid State 2019, 61, 1074–1085. [Google Scholar] [CrossRef]
- Kishimoto, T.; Oku, T.; Suzuki, A.; Ueoka, N. Additive effects of guanidinium iodide on CH3NH3PbI3 perovskite solar cells. Phys. Status Solidi (a) 2021, 218, 2100396. [Google Scholar] [CrossRef]
- Suzuki, A.; Oku, T. Effects of mixed-valence states of Eu-doped FAPbI3 perovskite crystals studied by first-principles calculation. Mater. Adv. 2021, 2, 2609–2616. [Google Scholar] [CrossRef]
- Suzuki, A.; Oku, T. First-principles calculation study of electronic structures of alkali metals (Li, K, Na and Rb)-incorporated formamidinium lead halide perovskite compounds. Appl. Surf. Sci. 2019, 483, 912–921. [Google Scholar] [CrossRef]
- Okumura, R.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of adding alkali metals and organic cations to cu-based perovskite solar cells. Appl. Sci. 2022, 12, 1710. [Google Scholar] [CrossRef]
- Enomoto, A.; Suzuki, A.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of Cu, K and guanidinium addition to CH3NH3PbI3 perovskite solar cells. J. Electron. Mater. 2022, 51, 4317–4328. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).